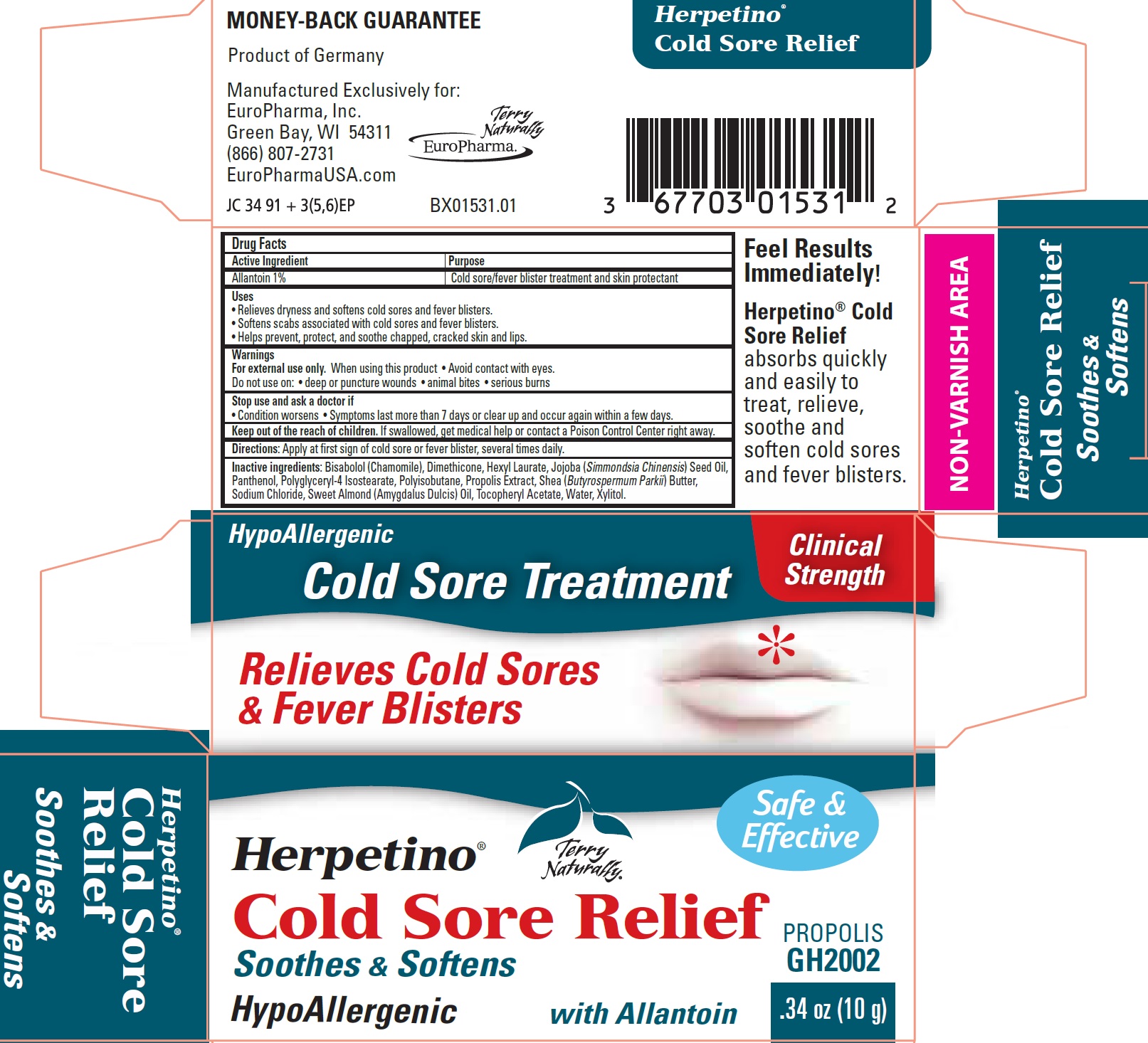
HERPETINO COLD SORE RELIEF TERRY NATURALLY BRAND- allantoin cream
EuroPharma, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Herpetino Cold Sore Relief Terry Naturally Brand
Uses
• Relieves dryness and softens cold sores and fever blisters.
• Softens scabs associated with cold sores and fever blisters.
• Helps prevent, protect, and soothe chapped, cracked skin and lips.
Warnings
For external use only.
Inactive ingredients:
Bisabolol (Chamomile), Dimethicone, Hexyl Laurate, Jojoba (Simmondsia Chinensis) Seed Oil, Panthenol, Polyglyceryl-4 Isostearate, Polyisobutane, Propolis Extract, Shea (Butyrospermum Parkii) Butter, Sodium Chloride, Sweet Almond (Amygdalus Dulcis) Oil, Tocopheryl Acetate, Water, Xylitol.
| HERPETINO COLD SORE RELIEF TERRY NATURALLY BRAND
allantoin cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - EuroPharma, Inc (060446577) |
| Registrant - EuroPharma, Inc (060446577) |