GUAIFENESIN EXTENDED-RELEASE- guaifenesin tablet, extended release
Guardian Pharmaceuticals, LLC.
----------
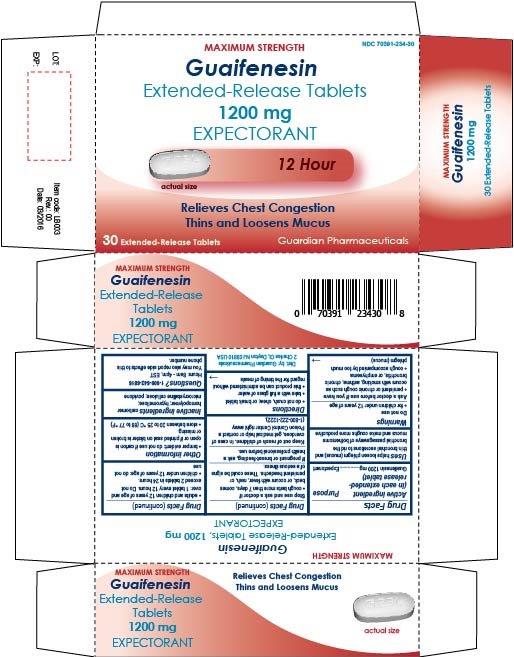
Guaifenesin Extended-Release Tablets, 600 mg and 1200 mg
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for the timing of meals
- adults and children 12 years of age and over: 1 or 2 tablets every 12 hours. Do not exceed 4 tablets in 24 hours.
- children under 12 years of age: do not use
Other information
- tamper evident: do not use if carton is open or if printed seal on blister is broken or missing
- store between 20 to 25 °C (68 to 77 °F)
Questions?
1-609-642-6816
Hours: 8am - 4pm, EST
You may also report side effects to this phone number.
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for the timing of meals
- adults and children 12 years of age and over: 1 tablet every 12 hours. Do not exceed 2 tablets in 24 hours.
- children under 12 years of age: do not use
Other information
- tamper evident: do not use if carton is open or if printed seal on blister is broken or missing
- store between 20 to 25 °C (68 to 77 °F)
Questions?
1-609-642-6816
Hours: 8am - 4pm, EST
You may also report side effects to this phone number.
| GUAIFENESIN EXTENDED-RELEASE
guaifenesin tablet, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| GUAIFENESIN EXTENDED-RELEASE
guaifenesin tablet, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Guardian Pharmaceuticals, LLC. (080059401) |
| Registrant - Guardian Drug Company (119210276) |
Revised: 10/2017
Document Id: 5ace6f16-3839-02c7-e053-2991aa0acdd8
Set id: c257a7d4-c245-4b66-a075-7890c6fec02a
Version: 6
Effective Time: 20171005
Guardian Pharmaceuticals, LLC.