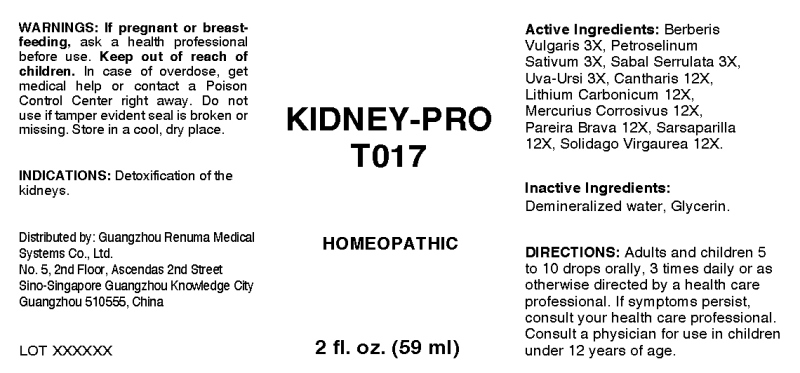
KIDNEY-PRO T017- berberis vulgaris, petroselinum sativum, sabal serrulata, uva ursi, cantharis, lithium carbonicum, mercurius corrosivus, pareira brava, sarsaparilla (smilax regelii), solidago virgaurea liquid
Guangzhou Renuma Medical Systems Co., Ltd
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
ACTIVE INGREDIENTS:
Berberis Vulgaris 3X, Petroselinum Sativum 3X, Sabal Serrulata 3X, Uva Ursi 3X, Cantharis 12X, Lithium Carbonicum 12X, Mercurius Corrosivus 12X, Pareira Brava 12X, Sarsaparilla (Smilax Regelii) 12X, Solidago Virgaurea 12X.
WARNINGS:
If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist, consult your health care professional. Consult a physician for use in children under 12 years of age.
| KIDNEY-PRO
T017
berberis vulgaris, petroselinum sativum, sabal serrulata, uva ursi, cantharis, lithium carbonicum, mercurius corrosivus, pareira brava, sarsaparilla (smilax regelii), solidago virgaurea liquid |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Guangzhou Renuma Medical Systems Co., Ltd (544538706) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(71742-0011) , api manufacture(71742-0011) , label(71742-0011) , pack(71742-0011) | |