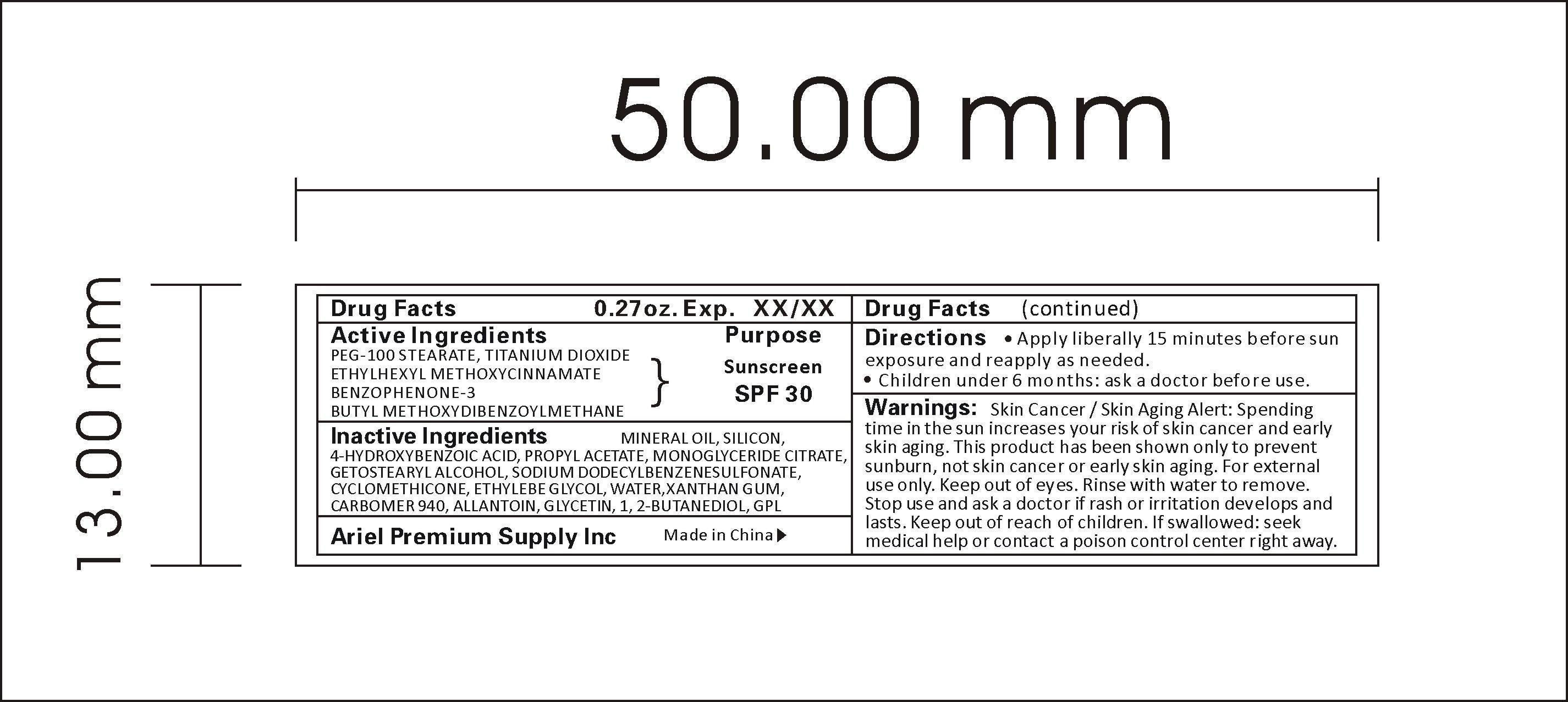
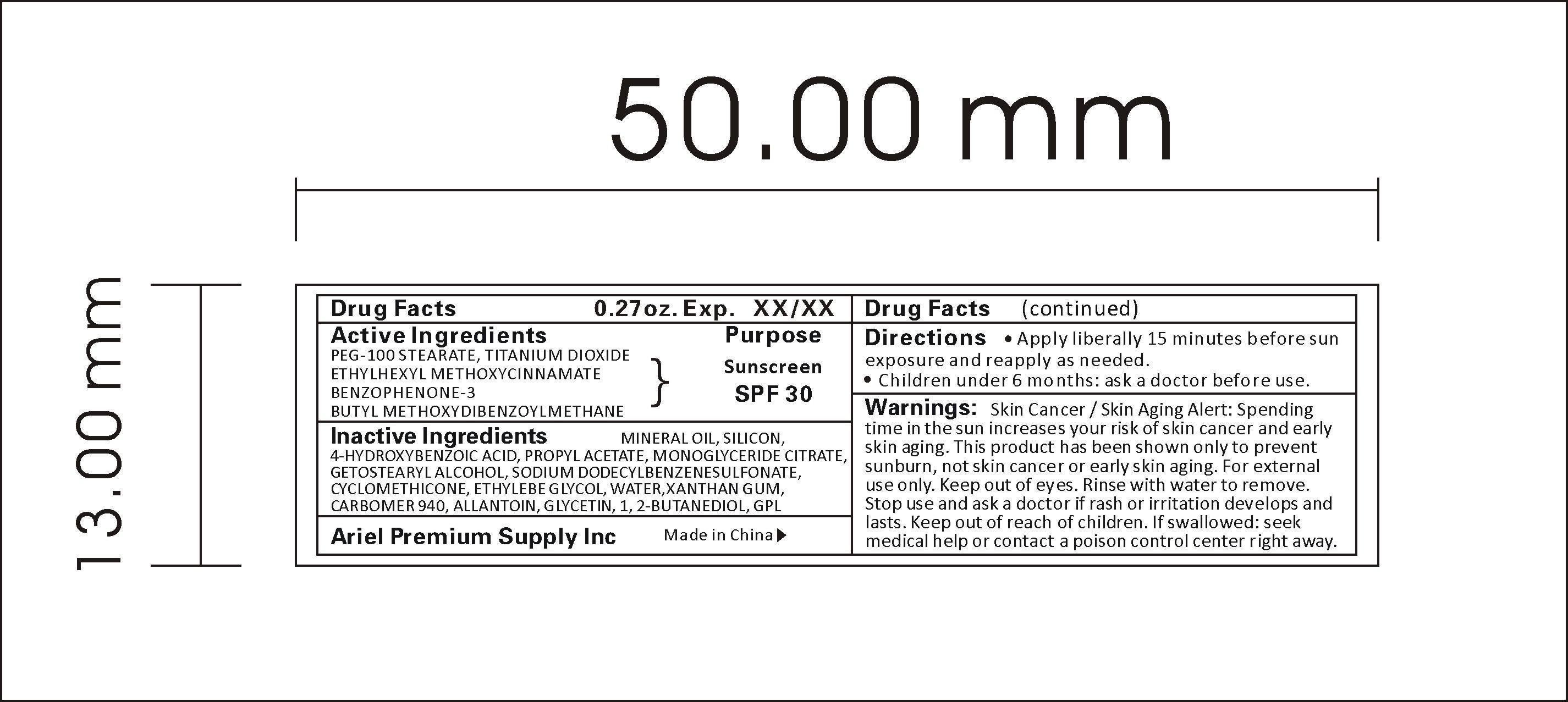
Label: SUNSCREEN SPF30 liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 47993-216-01, 47993-216-02, 47993-216-03, 47993-216-04, view more47993-216-05, 47993-216-06 - Packager: NINGBO JIANGBEI OCEAN STAR TRADING CO.,LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 18, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
WARNINGS
Warnings:
Skin Cancer / Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging. For external use only. Keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash or irritation develops and lasts. Keep out of reach of children. If swallowed: seek medical help or contact a poison control center right away.
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUNSCREEN SPF30
sunscreen spf30 liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47993-216 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEG-100 STEARATE (UNII: YD01N1999R) (PEG-100 STEARATE - UNII:YD01N1999R) PEG-100 STEARATE 2.5 g in 100 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4 g in 100 g ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) (ETHYLHEXYL METHOXYCRYLENE - UNII:S3KFG6Q5X8) ETHYLHEXYL METHOXYCRYLENE 1.25 g in 100 g BENZOPHENONE (UNII: 701M4TTV9O) (BENZOPHENONE - UNII:701M4TTV9O) BENZOPHENONE 0.5 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.75 g in 100 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) 5 g in 100 g SILICON (UNII: Z4152N8IUI) 1 g in 100 g 4-HYDROXYBENZOIC ACID (UNII: JG8Z55Y12H) 0.2 g in 100 g PROPYL ACETATE (UNII: 4AWM8C91G6) 0.1 g in 100 g GLYCERYL MONOOLEATE CITRATE (UNII: NLE5KIG74K) 1.5 g in 100 g CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) 2 g in 100 g SODIUM DODECYLBENZENESULFONATE (UNII: 554127163Y) 5 g in 100 g CYCLOMETHICONE (UNII: NMQ347994Z) 2 g in 100 g ETHYLENE GLYCOL (UNII: FC72KVT52F) 0.8 g in 100 g WATER (UNII: 059QF0KO0R) 59.7 g in 100 g XANTHAN GUM (UNII: TTV12P4NEE) 0.25 g in 100 g CARBOMER 940 (UNII: 4Q93RCW27E) 10 g in 100 g ALLANTOIN (UNII: 344S277G0Z) 0.3 g in 100 g GLYCERIN (UNII: PDC6A3C0OX) 2 g in 100 g 1,2-BUTANEDIOL (UNII: RUN0H01QEU) 3 g in 100 g DMDM HYDANTOIN (UNII: BYR0546TOW) 0.15 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47993-216-01 10 g in 1 TUBE; Type 0: Not a Combination Product 06/01/2015 2 NDC:47993-216-02 15 g in 1 TUBE; Type 0: Not a Combination Product 06/01/2015 3 NDC:47993-216-03 20 g in 1 TUBE; Type 0: Not a Combination Product 06/01/2015 4 NDC:47993-216-04 30 g in 1 TUBE; Type 0: Not a Combination Product 06/01/2015 5 NDC:47993-216-05 50 g in 1 TUBE; Type 0: Not a Combination Product 06/01/2015 6 NDC:47993-216-06 60 g in 1 TUBE; Type 0: Not a Combination Product 06/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/20/2015 Labeler - NINGBO JIANGBEI OCEAN STAR TRADING CO.,LTD (529334491) Registrant - NINGBO JIANGBEI OCEAN STAR TRADING CO.,LTD (529334491) Establishment Name Address ID/FEI Business Operations NINGBO OCEANSTAR CHEMICAL PRODUCTS CO.,LTD 544493972 manufacture(47993-216)