LYM D HP- arsenicum album, chelidonium majus, coccus cacti, echinacea angustifolia, hydrastis canadensis, lycopodium clavatum, lym d nosode, phosphorus, rhus toxicodendron, taraxacum officinale, thyroidinum, trifolium pratense, urtica dioica, thymus, nasturtium aquaticum, liquid
Apotheca Company
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
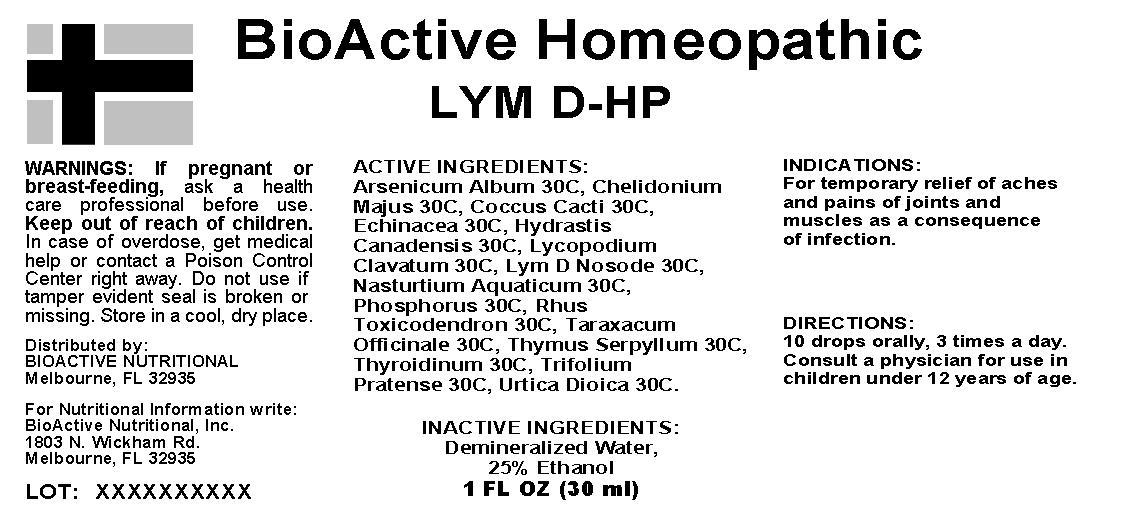
ACTIVE INGREDIENTS: Arsenicum album 30C, Chelidonium majus 30C, Coccus cacti 30C, Echinacea angustifolia 30C, Hydrastis canadensis 30C, Lycopodium clavatum 30C, Lym D Nosode 30C, Phosphorus 30X, Rhus toxicodendron 30C, Taraxacum officinale 30C, Thyroidinum 30C, Trifolium pratense 30C, Urtica dioica 30C, Thymus 30C, Nasturtium aquaticum 30C.
INDICATIONS: For temporary relief of aches and pains of joints and muscles as a consequence of infection.
WARNINGS: If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS: 10 drops orally, 3 times a day. Consult a physician for use in children under 12 years of age.
INDICATIONS & USAGE:
For temporary relief of aches and pains of joints and muscles as a consequence of infection.
| LYM D
HP
arsenicum album, chelidonium majus, coccus cacti, echinacea angustifolia, hydrastis canadensis, lycopodium clavatum, lym d nosode, phosphorus, rhus toxicodendron, taraxacum officinale, thyroidinum, trifolium pratense, urtica dioica, thymus, nasturtium aquaticum, liquid |
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Apotheca Company (844330915) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(57520-0518) , api manufacture(57520-0518) , label(57520-0518) , pack(57520-0518) | |