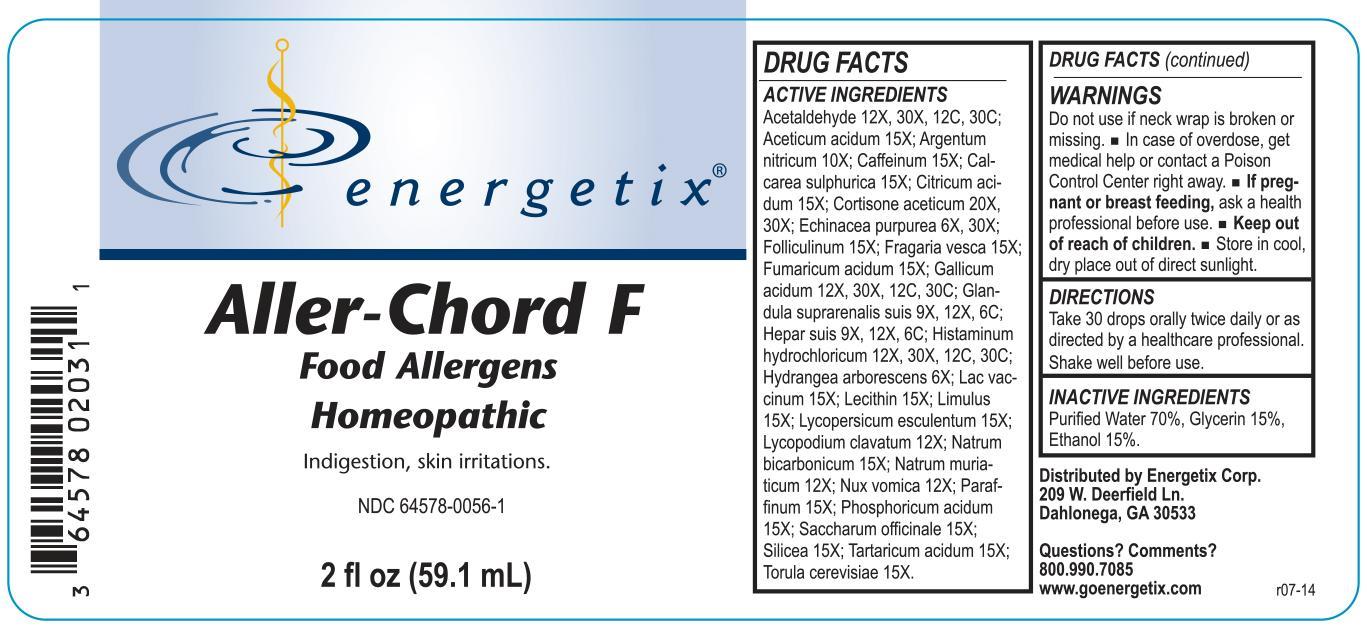
Label: ALLER-CHORD FOOD- homeopathic liquid liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 64578-0056-1 - Packager: Energetix Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 2, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
ACTIVE INGREDIENT
Acetaldehyde 12X, 30X, 12C, 30C; Aceticum acidum 15X; Argentum nitricum 10X; Caffeinum 15X; Calcarea sulphurica 15X; Citricum acidum 15X; Cortisone aceticum 20X, 30X; Echinacea purpurea 6X, 30X; Folliculinum 15X; Fragaria vesca 15X; Fumaricum acidum 15X; Gallicum acidum 12X, 30X, 12C, 30C; Glandula suprarenalis suis 9X, 12X, 6C; Hepar suis 9X, 12X, 6C; Histaminum hydrochloricum 12X, 30X, 12C, 30C; Hydrangea arborescens 6X; Lac vaccinum 15X; Lecithin 15X; Limulus 15X; Lycopersicum esculentum 15X; Lycopodium clavatum 12X; Natrum bicarbonicum 15X; Natrum muriaticum 12X; Nux vomica 12X; Paraffinum 15X; Phosphoricum acidum 15X; Saccharum officinale 15X; Silicea 15X; Tartaricum acidum 15X; Torula cerevisiae 15X
- WARNINGS
- DO NOT USE
- OVERDOSAGE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- QUESTIONS
- PURPOSE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
-
INGREDIENTS AND APPEARANCE
ALLER-CHORD FOOD
homeopathic liquid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64578-0056 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETALDEHYDE (UNII: GO1N1ZPR3B) (ACETALDEHYDE - UNII:GO1N1ZPR3B) ACETALDEHYDE 12 [hp_X] in 59.1 mL SUS SCROFA ADRENAL GLAND (UNII: 398IYQ16YV) (SUS SCROFA ADRENAL GLAND - UNII:398IYQ16YV) SUS SCROFA ADRENAL GLAND 9 [hp_X] in 59.1 mL SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 10 [hp_X] in 59.1 mL CORTISONE ACETATE (UNII: 883WKN7W8X) (CORTISONE - UNII:V27W9254FZ) CORTISONE ACETATE 20 [hp_X] in 59.1 mL GALLIC ACID MONOHYDRATE (UNII: 48339473OT) (GALLIC ACID - UNII:632XD903SP) GALLIC ACID MONOHYDRATE 12 [hp_X] in 59.1 mL HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 12 [hp_X] in 59.1 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 6 [hp_X] in 59.1 mL HYDRANGEA ARBORESCENS ROOT (UNII: SFK828Q2DE) (HYDRANGEA ARBORESCENS ROOT - UNII:SFK828Q2DE) HYDRANGEA ARBORESCENS ROOT 6 [hp_X] in 59.1 mL PORK LIVER (UNII: 6EC706HI7F) (PORK LIVER - UNII:6EC706HI7F) PORK LIVER 9 [hp_X] in 59.1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 12 [hp_X] in 59.1 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 12 [hp_X] in 59.1 mL ACETIC ACID (UNII: Q40Q9N063P) (ACETIC ACID - UNII:Q40Q9N063P) ACETIC ACID 15 [hp_X] in 59.1 mL CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 15 [hp_X] in 59.1 mL CALCIUM SULFATE ANHYDROUS (UNII: E934B3V59H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFATE ANHYDROUS 15 [hp_X] in 59.1 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 15 [hp_X] in 59.1 mL FUMARIC ACID (UNII: 88XHZ13131) (FUMARIC ACID - UNII:88XHZ13131) FUMARIC ACID 15 [hp_X] in 59.1 mL COW MILK (UNII: 917J3173FT) (COW MILK - UNII:917J3173FT) COW MILK 15 [hp_X] in 59.1 mL EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) (EGG PHOSPHOLIPIDS - UNII:1Z74184RGV) EGG PHOSPHOLIPIDS 15 [hp_X] in 59.1 mL SODIUM BICARBONATE (UNII: 8MDF5V39QO) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM BICARBONATE 15 [hp_X] in 59.1 mL SUCROSE (UNII: C151H8M554) (SUCROSE - UNII:C151H8M554) SUCROSE 15 [hp_X] in 59.1 mL TARTARIC ACID (UNII: W4888I119H) (TARTARIC ACID - UNII:W4888I119H) TARTARIC ACID 15 [hp_X] in 59.1 mL SACCHAROMYCES CEREVISIAE (UNII: 978D8U419H) (SACCHAROMYCES CEREVISIAE - UNII:978D8U419H) SACCHAROMYCES CEREVISIAE 15 [hp_X] in 59.1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 12 [hp_X] in 59.1 mL PARAFFIN (UNII: I9O0E3H2ZE) (PARAFFIN - UNII:I9O0E3H2ZE) PARAFFIN 15 [hp_X] in 59.1 mL ESTRONE (UNII: 2DI9HA706A) (ESTRONE - UNII:2DI9HA706A) ESTRONE 15 [hp_X] in 59.1 mL PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 15 [hp_X] in 59.1 mL ALPINE STRAWBERRY (UNII: CG6IX3GCMU) (ALPINE STRAWBERRY - UNII:CG6IX3GCMU) ALPINE STRAWBERRY 15 [hp_X] in 59.1 mL LIMULUS POLYPHEMUS (UNII: 3R8874KEYH) (LIMULUS POLYPHEMUS - UNII:3R8874KEYH) LIMULUS POLYPHEMUS 15 [hp_X] in 59.1 mL SOLANUM LYCOPERSICUM (UNII: 0243Q4990L) (SOLANUM LYCOPERSICUM - UNII:0243Q4990L) SOLANUM LYCOPERSICUM 15 [hp_X] in 59.1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 15 [hp_X] in 59.1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 41.36 mL in 59.1 mL GLYCERIN (UNII: PDC6A3C0OX) 8.86 mL in 59.1 mL WATER (UNII: 059QF0KO0R) 8.86 mL in 59.1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64578-0056-1 59.1 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/08/2014 Labeler - Energetix Corp (969572502) Establishment Name Address ID/FEI Business Operations Terra Botanica LLC 963736785 manufacture(64578-0056) , label(64578-0056) , pack(64578-0056)