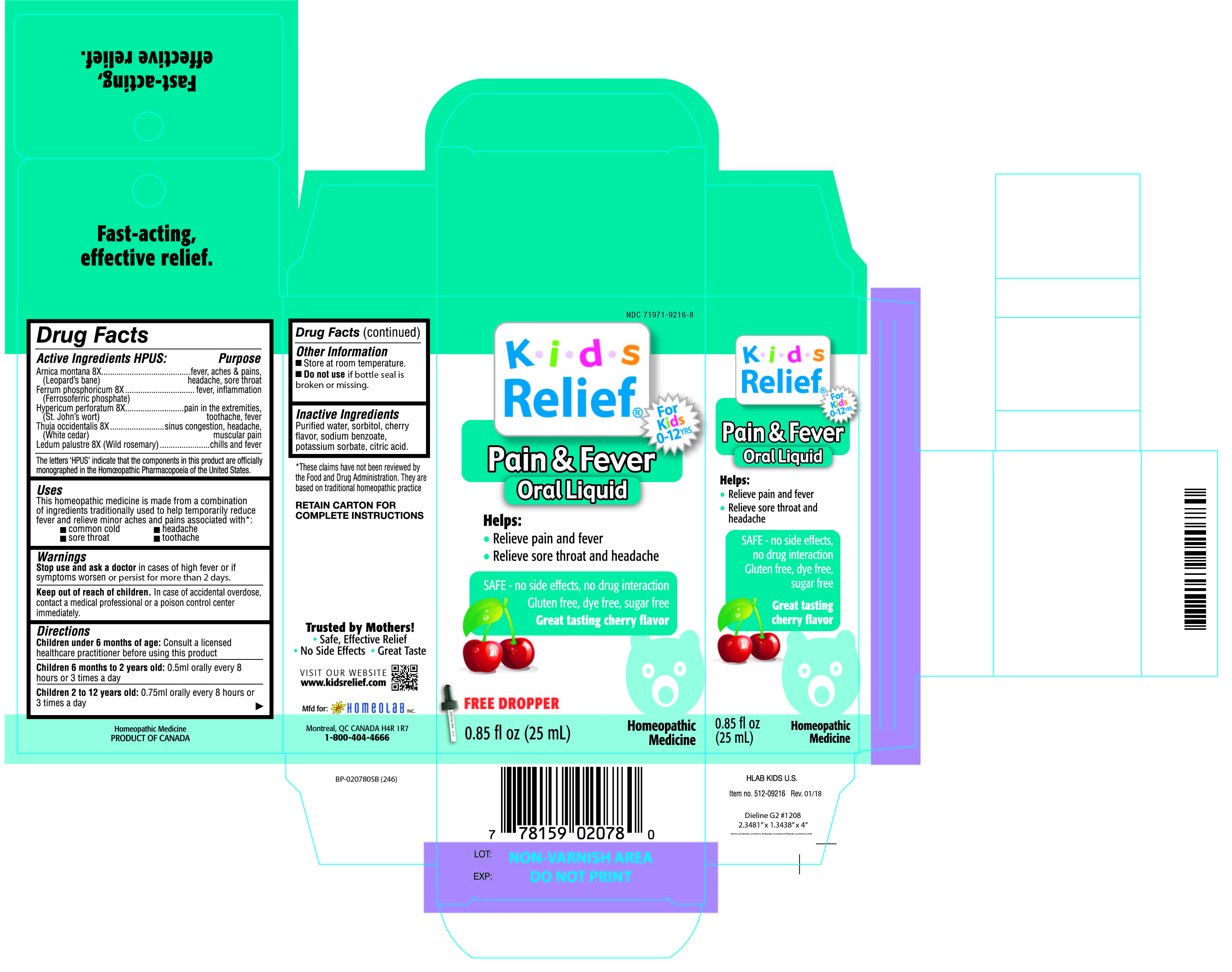
Label: KIDS RELIEF- arnica montana, ferrum phosphoricum, hypericum perforatum, thuja occidentalis, ledum palustre liquid
- NDC Code(s): 71971-9216-8
- Packager: Homeolab International (Canada) inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 16, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients HPUS:
Arnica montana 8X
(Leopard's bane)
Ferrum phosphoricum 8X
(Ferrosoferric phosphate)
Hypericum perforatum 8X
(St. John's wort)
Thuja occidentalis 8X
(White cedar)
Ledum palustre 8X (Wild rosemary)
The letters 'HPUS' indicate that the component in this product is officially
monographed in the Homeopathic Pharmacopoeia of the United States.
-
PURPOSE
Purpose
Arnica montana 8X.............................................fever, aches & pains, headache, sore throat
(Leopard's bane)
Ferrum phosphoricum 8X.....................................fever, inflammation
(Ferrosoferric phosphate)
Hypericum perforatum 8X....................................pain in the extremeties, toothache, fever
(St. John's wort)
Thuja occidentalis 8X..........................................sinus congestion, headache, muscular pain
(White cedar)
Ledum palustre 8X (Wild rosemary).....................chills and fever
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- Kids Relief Pain & Fever Oral Liquid
- Kids Relief Pain & Fever Oral Liquid
-
INGREDIENTS AND APPEARANCE
KIDS RELIEF
arnica montana, ferrum phosphoricum, hypericum perforatum, thuja occidentalis, ledum palustre liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71971-9216 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 8 [hp_X] in 25 mL FERRUM PHOSPHORICUM (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERRUM PHOSPHORICUM 8 [hp_X] in 25 mL HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 8 [hp_X] in 25 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 8 [hp_X] in 25 mL RHODODENDRON TOMENTOSUM LEAFY TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) RHODODENDRON TOMENTOSUM LEAFY TWIG 8 [hp_X] in 25 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRIC ACID ACETATE (UNII: DSO12WL7AU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71971-9216-8 1 in 1 CARTON 03/03/2017 1 25 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/03/2017 Labeler - Homeolab International (Canada) inc (203639455)