TOTAL AGE DEFY SPF 15 SUNSCREEN- ensulizole, octinoxate, octisalate, octocrylene, oxybenzone, titanium dioxide cream
S+
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
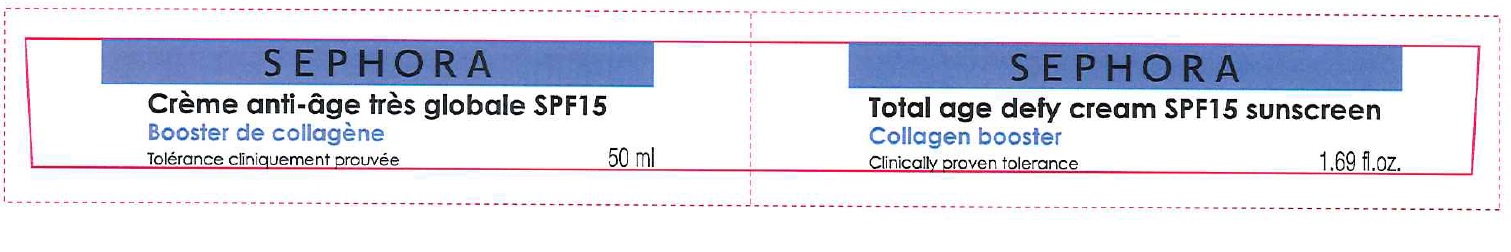
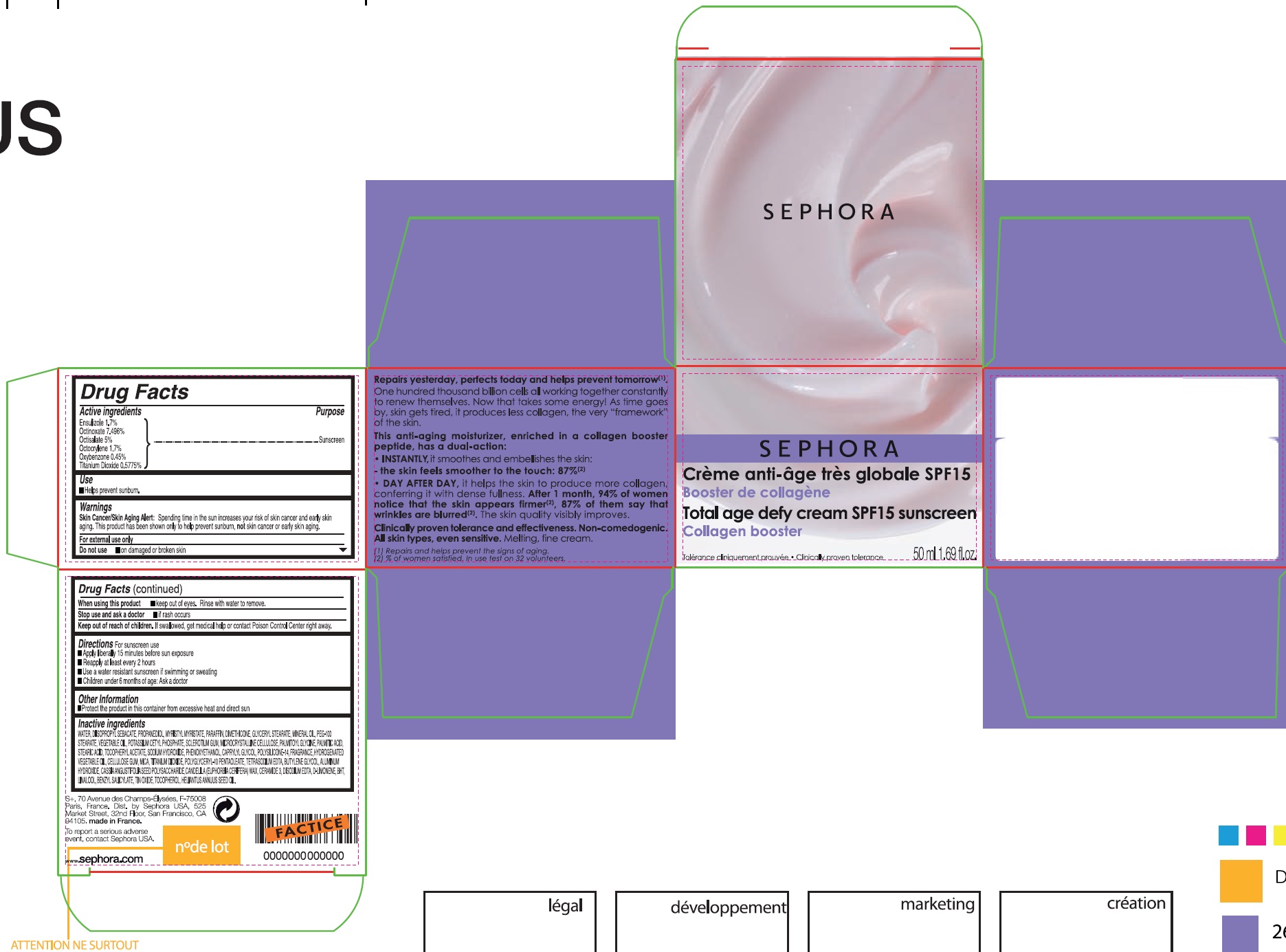
CREME ANTI-AGE TRES GLOBALE SPF 15/ TOTAL AGE DEFY CREAM SPF 15 SUNSCREEN
Active ingredients
Ensulizole 1.7%
Octinoxate 7.496%
Octisalate 5%
Octocrylene 1.7%
Oxybenzone 0.45%
Titanium Dioxide 0.5775%
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increses your skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only
Directions For sunscreen use
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months of age: Ask a doctor
Inactive ingredients
WATER, DIISOPROPYL SEBACATE, PROPANEDIOL, MYRISTYL MYRISTATE, PARAFFIN, DIMETHICONE, GLYCERYL STEARATE, MINERAL OIL, PEG-100 STEARATE, VEGETABLE OIL, POTASSIUM CETYL PHOSPHATE, SCLEROTIUM GUM, MICROCRYSTALLINE CELLULOSE, PALMITOYL GLYCINE, PALMITIC ACID, STEARIC ACID, TOCOPHERYL ACETATE, SODIUM HYDROXIDE, PHENOXYETHANOL, CAPRYLYL GLYCOL, POLYSILICONE-14, FRAGRANCE, HYDROGENATED VEGETABLE OIL, CELLULOSE GUM, MICA, TITANIUM DIOXIDE, POLYGLYCERYL-10 PENTAOLEATE, TETRASODIUM EDTA, BUTYLENE GLYCOL, ALIMINIUM HYDROXIDE, CASSIA ANGUSTIFOLIA SEED POLYSACCHARIDE, CANDELILA (EUPHORBIA CERIFERA) WAX, CERAMIDE 3, DISODIUM EDTA, D-LIMONENE, BHT, LINALOOL, BENZYL SALICYLATE, TIN OXIDE, HELIANTUS ANNUUS SEED OIL.
| TOTAL AGE DEFY SPF 15 SUNSCREEN
ensulizole, octinoxate, octisalate, octocrylene, oxybenzone, titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - S+ (572406531) |