DIABET AID- capsicum oleoresin lotion
Insight Pharmaceuticals LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DIABET AID
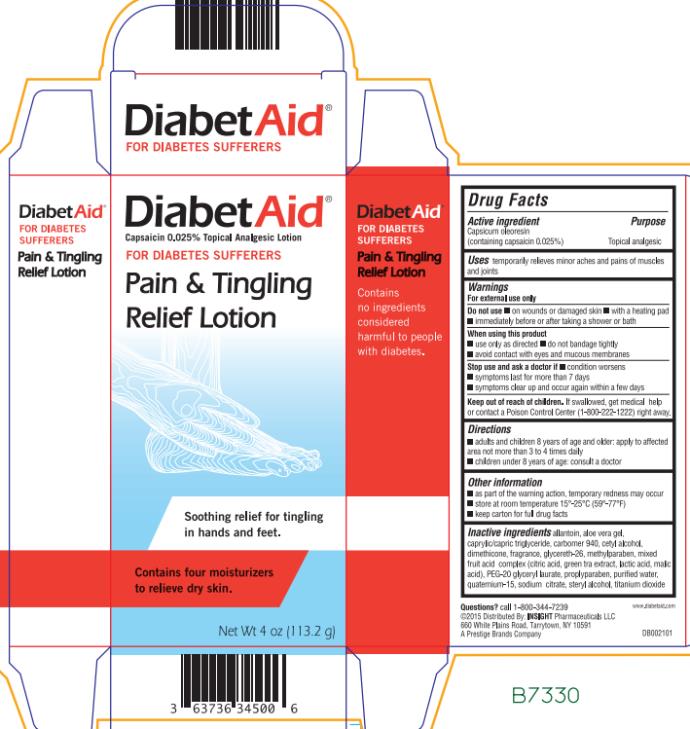
Warnings
For external use only
Do not use
- on wounds or damaged skin
- with a heating pad
- immediately before or after taking a shower or bath
When using this product
- avoid contact with eyes
- do not bandage tightly
- do not apply to wounds or damaged skin
Directions
| Adults and children 8 years of age and older | apply to affected area not more than 3 to 4 times daily |
| Children under 8 years of age | consult a doctor |
Other information
- as part of the warming action, temporary redness may occur
- store at room temperature 15° - 25°C (59° - 77°F)
- keep carton for full drug facts
Inactive ingredients
allantoin, aloe vera gel, caprylic/capric triglyceride, carbomer, cetyl alcohol, dimethicone, fragrance, methylparaben, mixed fruit acid complex (citric acid, green tea extract, lactic acid, malic acid), PEG-20 glyceryl laurate, PEG-30 glyceryl laurate, propylparaben, purified water, quaternium-15, sodium citrate, stearyl alcohol, titanium dioxide.
| DIABET AID
capsicum oleoresin lotion |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Insight Pharmaceuticals LLC (055665422) |
Revised: 8/2023
Document Id: 7760deda-c1c5-4774-8015-84610aecbbc2
Set id: bd6a8947-1268-4db4-a602-fa41e476b9c4
Version: 4
Effective Time: 20230818
Insight Pharmaceuticals LLC