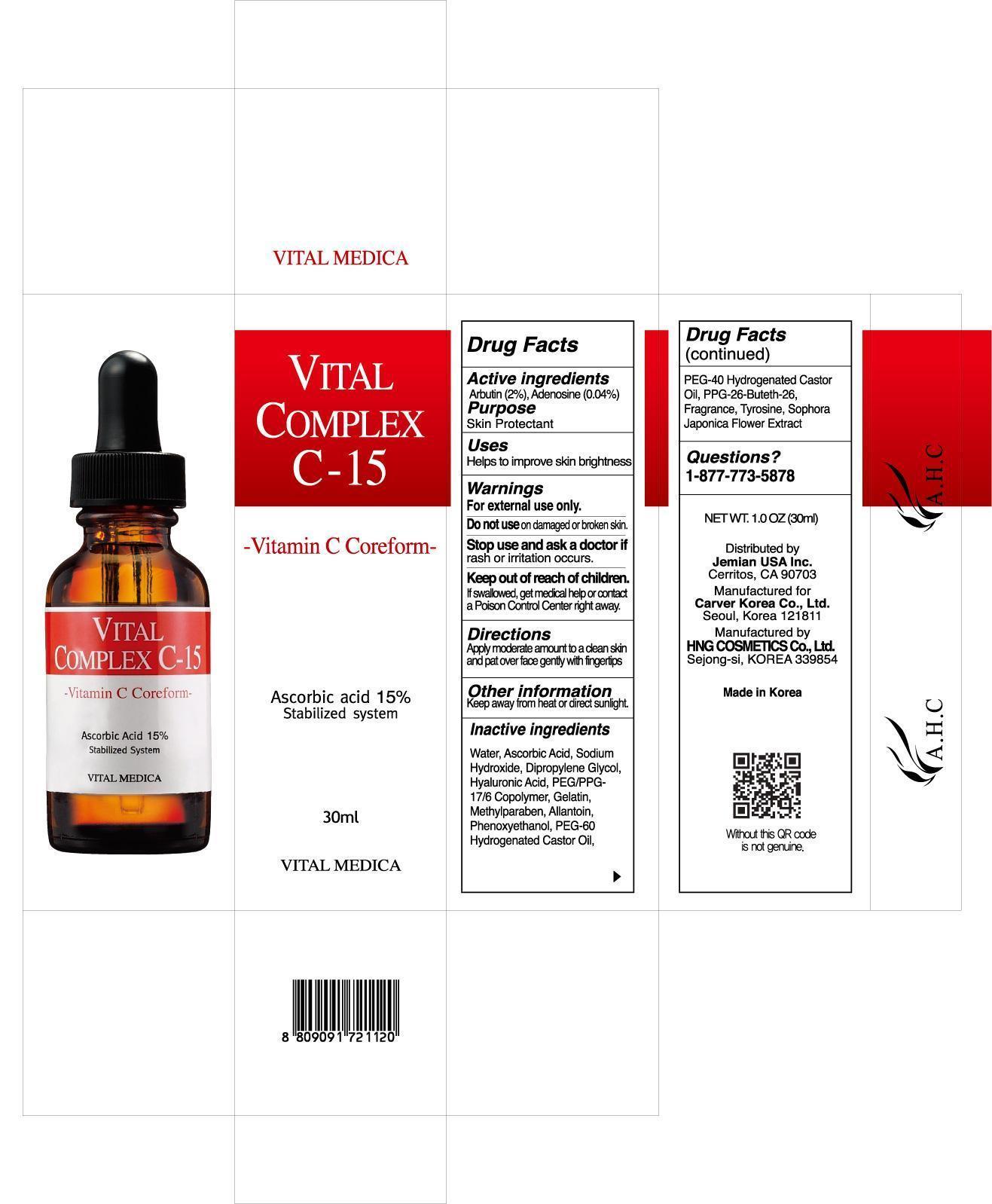
AHC VITAL COMPLEX C-15 AMPOULE- arbutin 2%, adenosine 0.04% liquid
Carver Korea Co.,Ltd
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
AHC Vital Complex C-15 Ampoule
| AHC VITAL COMPLEX C-15 AMPOULE
arbutin 2%, adenosine 0.04% liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Carver Korea Co.,Ltd (688442290) |
| Registrant - Jemian USA Inc. (078822273) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| HNG CO., LTD | 689851457 | manufacture(58930-040) | |
Revised: 5/2019
Document Id: 8ef25646-286e-4602-8348-1a9203611222
Set id: bd67e4ac-be0a-4bc8-9f00-a0d8b17b9c81
Version: 2
Effective Time: 20190520
Carver Korea Co.,Ltd