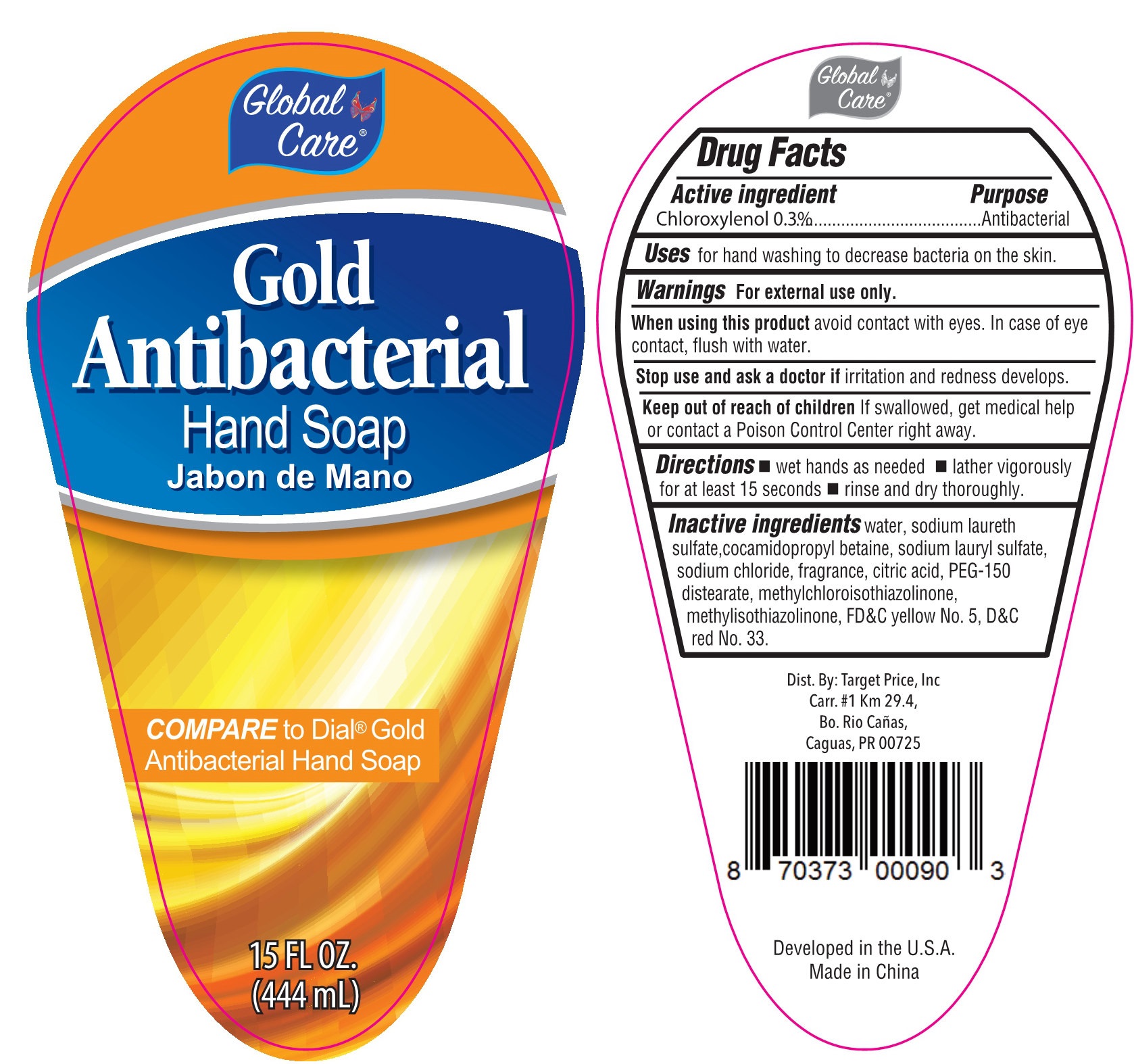
GOLD ANTIBACTERIAL HAND- chloroxylenol liquid
Target Price, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Gold Antibacterial Hand
| GOLD ANTIBACTERIAL HAND
chloroxylenol liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Target Price, Inc (081086884) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ningbo Liyuan Daily Chemical Products Co., Ltd | 530766098 | manufacture(72146-001) | |
Revised: 10/2021
Document Id: cd8b5621-4368-6567-e053-2a95a90a3a92
Set id: bcbac228-d8ef-4223-af5c-59f52d626a56
Version: 4
Effective Time: 20211004
Target Price, Inc