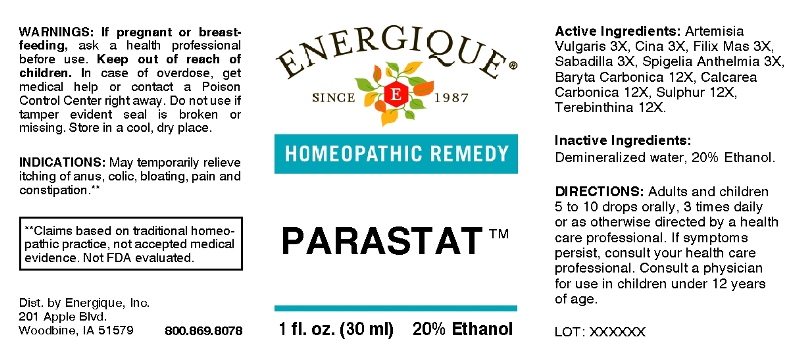
PARASTAT- artemisia vulgaris, cina, filix mas, sabadilla, spigelia anthelmia, baryta carbonica, calcarea carbonica, sulphur, terebinthina liquid
Energique, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
ACTIVE INGREDIENTS:
Artemisia Vulgaris 3X, Cina 3X, Filix Mas 3X, Sabadilla 3X, Spigelia Anthelmia 3X, Baryta Carbonica 12X, Calcarea Carbonica 12X, Sulphur 12X, Terebinthina 12X.
INDICATIONS:
For temporary relief of itching of anus, colic, bloating, pain and constipation.**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
Store in a cool, dry place.
DIRECTIONS:
Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist, consult your health care professional. Consult a physician for use in children under 12 years of age.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
| PARASTAT
artemisia vulgaris, cina, filix mas, sabadilla, spigelia anthelmia, baryta carbonica, calcarea carbonica, sulphur, terebinthina liquid |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - Energique, Inc. (789886132) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(44911-0034) , api manufacture(44911-0034) , label(44911-0034) , pack(44911-0034) | |