Label: STROVITE ONE CAPLETS- vitamin a, calcium pantothenate, ascorbic acid, cholecalciferol, .alpha.-tocopherol succinate, d-, thiamine, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, biotin, cyanocobalamin, selenium, magnesium oxide, zinc oxide, cupric sulfate, manganese, chromium, .alpha.-lipoic acid, and lutein tablet
- NDC Code(s): 0642-0207-03, 0642-0207-90
- Packager: Exeltis USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
COMPOSITION
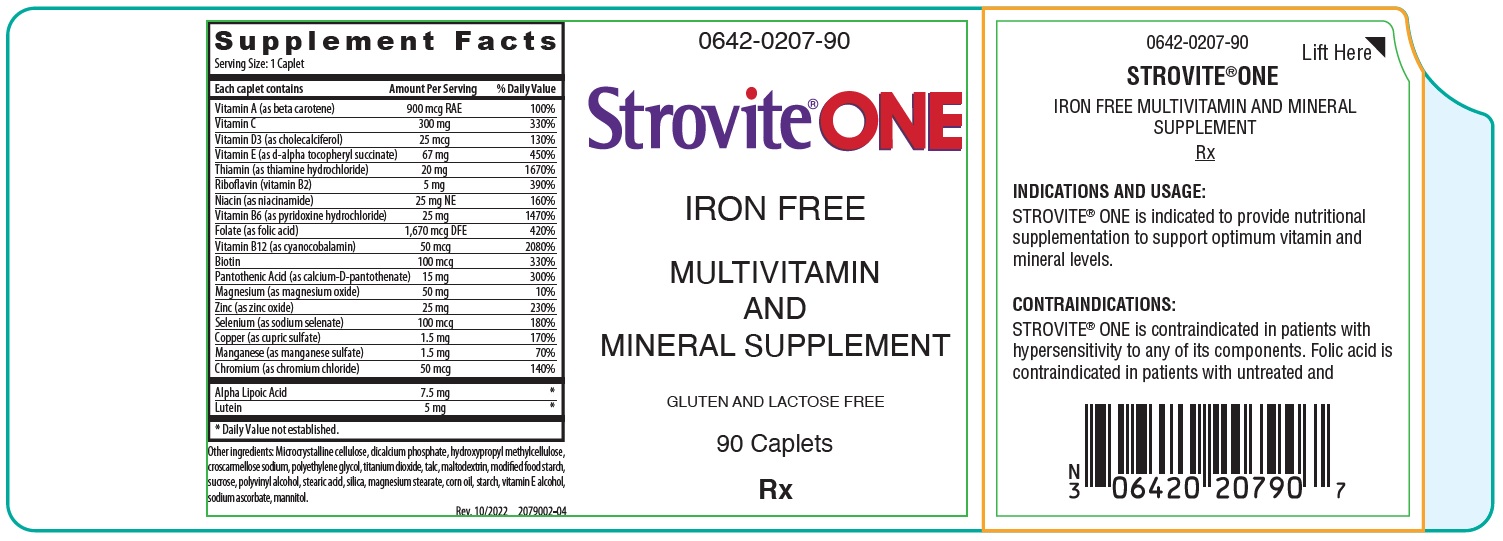
Each caplet contains: Vitamin A (as beta carotene) 900 mcg RAE Vitamin C 300 mg Vitamin D (as cholecalciferol) 25 mcg Vitamin E (as d-alpha tocopheryl succinate) 67 mg Thiamin (as thiamine hydrochloride) 20 mg Riboflavin (vitamin B2) 5 mg Niacin (as niacinamide) 25 mg NE Vitamin B6 (as pyridoxine hydrochloride) 25 mg Folate (as folic acid) 1,670 mcg DFE Vitamin B12 (as cyanocobalamin) 50 mcg Biotin 100 mcg Pantothenic Acid (as calcium-D-pantothenate) 15 mg Magnesium (as magnesium oxide) 50 mg Zinc (as zinc oxide) 25 mg Selenium (as sodium selenate) 100 mcg Copper (as cupric sulfate) 1.5 mg Manganese (as manganese sulfate) 1.5 mg Chromium (as chromium chloride) 50 mcg Alpha Lipoic Acid 7.5 mg Lutein 5 mg Other ingredients: Microcrystalline cellulose, dicalcium phosphate, hydroxypropyl methylcellulose, croscarmellose sodium, polyethylene glycol, titanium dioxide, talc, maltodextrin, modified food starch, sucrose, polyvinyl alcohol, stearic acid, silica, magnesium stearate, corn oil, starch, vitamin E alcohol, sodium ascorbate, mannitol.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
STROVITE ® ONE is contraindicated in patients with hypersensitivity to any of its components. Folic Acid is contraindicated in patients with untreated and uncomplicated pernicious anemia, and in those with anaphylactic sensitivity to folic acid.
Cyanocobalamin is contraindicated in patients with sensitivity to cobalt or to cyanocobalamin (Vitamin B12).
-
WARNING/PRECAUTIONS
Vitamin D supplementation should be used with caution in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. High doses of vitamin D can lead to elevated levels of calcium that reside in the blood and soft tissues. Bone pain, high blood pressure, formation of kidney stones, renal failure, and increased risk of heart disease can occur.
Folic acid, especially in doses above 0.1 mg daily, may obscure pernicious anemia, in that hematologic remission may occur while neurological manifestations remain progressive.
The use of folic acid doses above 1 mg daily may precipitate or exacerbate the neurological damage of vitamin B12 deficiency.
Avoid overdosage. Keep out of the reach of children.
-
PRECAUTIONS
Drug Interactions
High doses of folic acid may result in decreased serum levels of anticonvulsant drugs.
Vitamin D supplementation should not be given with large amounts of calcium in those with hypercalcemia or conditions that may lead to hyper-calcemia such as hyperparathyroidism and those who form calcium-containing kidney stones.
Zinc can inhibit the absorption of certain antibiotics; take at least 2 hours apart to minimize interactions. Consult appropriate references for additional specific vitamin-drug interactions.
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 90 Caplet Bottle Label
-
INGREDIENTS AND APPEARANCE
STROVITE ONE CAPLETS
vitamin a, calcium pantothenate, ascorbic acid, cholecalciferol, .alpha.-tocopherol succinate, d-, thiamine, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, biotin, cyanocobalamin, selenium, magnesium oxide, zinc oxide, cupric sulfate, manganese, chromium, .alpha.-lipoic acid, and lutein tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0642-0207 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANGANESE (UNII: 42Z2K6ZL8P) (MANGANESE - UNII:42Z2K6ZL8P) MANGANESE 1.5 mg VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 900 ug CALCIUM PANTOTHENATE (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737, CALCIUM CATION - UNII:2M83C4R6ZB) PANTOTHENIC ACID 7.5 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 300 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 25 ug .ALPHA.-TOCOPHEROL SUCCINATE, D- (UNII: LU4B53JYVE) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL SUCCINATE, D- 45 ug THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 20 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 5 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 25 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 25 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1700 ug BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 100 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 50 ug SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 100 ug MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 50 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 25 mg CUPRIC SULFATE (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 1.5 mg CHROMIUM (UNII: 0R0008Q3JB) (CHROMIUM - UNII:0R0008Q3JB) CHROMIUM 50 ug ALPHA LIPOIC ACID (UNII: 73Y7P0K73Y) (ALPHA LIPOIC ACID - UNII:73Y7P0K73Y) ALPHA LIPOIC ACID 15 mg LUTEIN (UNII: X72A60C9MT) (LUTEIN - UNII:X72A60C9MT) LUTEIN 5 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) STARCH, CORN (UNII: O8232NY3SJ) SOYBEAN OIL (UNII: 241ATL177A) TALC (UNII: 7SEV7J4R1U) SUCROSE (UNII: C151H8M554) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM ASCORBATE (UNII: S033EH8359) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) Product Characteristics Color white Score no score Shape OVAL Size 19mm Flavor Imprint Code EV0207 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0642-0207-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 05/04/2001 2 NDC:0642-0207-03 1 in 1 BOX 05/04/2001 2 3 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/04/2001 Labeler - Exeltis USA, Inc. (071170534)