Label: NICOTINE COATED FRUIT FLAVOR- nicotine polacrilex gum, chewing
-
Contains inactivated NDC Code(s)
NDC Code(s): 68196-015-01, 68196-016-01 - Packager: Sam's West, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 13, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each chewing piece)
- Purpose
- Use
-
Warnings
Pregnancy/Breastfeeding
If you are pregnant or breast feeding, only use this medicine on the advice of your health care provider. Smoking can seriously harm your child. Try to stop smoking without using any nicotine replacement medicine. This medicine is believed to be safer than smoking. However, the risks to your child from this medicine are not fully known.
Ask a doctor before use if you have
- a sodium-restricted diet
- heart disease, recent heart attack, or irregular heartbeat. Nicotine can increase your heart rate.
- high blood pressure not controlled with medication. Nicotine can increase blood pressure.
- stomach ulcer or diabetes
- history of seizures
Ask a doctor or pharmacist before use if you are
- using a non-nicotine stop smoking drug
- taking prescription medicine for depression or asthma. Your prescription dose may need to be adjusted.
Stop use and ask a doctor if
- mouth, teeth, or jaw problems occur
- irregular heartbeat or palpitations occur
- you get symptoms of nicotine overdose such as nausea, vomiting, dizziness, diarrhea, weakness, and rapid heartbeat
- you have symptoms of an allergic reaction (such as difficulty breathing or rash)
-
Directions (2 mg)
- if you are under 18 years of age, ask a doctor before use
- before using this product, read the enclosed User's Guide for complete instructions and other important information
- begin using the gum on your quit day
- if you smoke your first cigarette within 30 minutes of waking up, use 4 mg nicotine gum
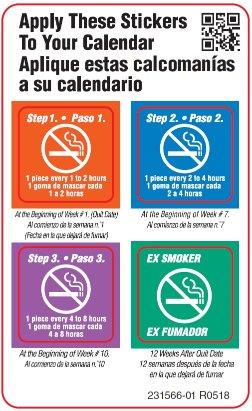
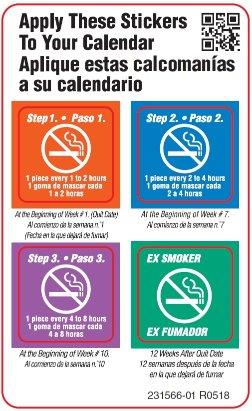
- if you smoke your first cigarette more than 30 minutes after waking up, use 2 mg nicotine gum according to the following 12 week schedule:
Weeks 1 to 6 Weeks 7 to 9 Weeks 10 to 12 1 piece every
1 to 2 hours1 piece every
2 to 4 hours1 piece every
4 to 8 hours- nicotine gum is a medicine and must be used in a certain way to get the best results
- chew the gum slowly until it tingles. Then park it between your cheek and gum. When the tingle is gone, begin chewing again, until the tingle returns.
- repeat this process until most of the tingle is gone (about 30 minutes)
- do not eat or drink for 15 minutes before chewing the nicotine gum, or while chewing a piece
- to improve your chances of quitting, use at least 9 pieces per day for the first 6 weeks
- if you experience strong or frequent cravings, you may use a second piece within the hour. However, do not continuously use one piece after another since this may cause you hiccups, heartburn, nausea, or other side effects.
- do not use more than 24 pieces a day
- it is important to complete treatment. If you feel you need to use the gum for a longer period to keep from smoking, talk to your health care provider.
-
Directions (4 mg)
- if you are under 18 years of age, ask a doctor before use
- before using this product, read the enclosed User’s Guide for complete insructions and other important information
- begin using the gum on your quit day
- if you smoke your first cigarette more than 30 minutes after waking up, use 2 mg nicotine gum
- if you smoke your first cigarette within 30 minutes of waking up, use 4 mg nicotine gum according to the following 12 week schedule:
Weeks 1 to 6 Weeks 7 to 9 Weeks 10 to 12 1 piece every
1 to 2 hours1 piece every
2 to 4 hours1 piece every
4 to 8 hours- nicotine gum is a medicine and must be used in a certain way to get the best results
- chew the gum slowly until it tingles. Then park it between your cheek and gum. When the tingle is gone, begin chewing again, until the tingle returns.
- repeat this process until most of the tingle is gone (about 30 minutes)
- do not eat or drink for 15 minutes before chewing the nicotine gum, or while chewing a piece
- to improve your chances of quitting, use at least 9 pieces per day for the first 6 weeks
- if you experience strong or frequent cravings, you may use a second piece within the hour. However, do not continuously use one piece after another since this may cause you hiccups, heartburn, nausea, or other side effects.
- do not use more than 24 pieces a day
- it is important to complete treatment. If you feel you need to use the gum for a longer period to keep from smoking, talk to your health care provider.
- Other information
- Inactive ingredients (2 mg)
- Inactive ingredients (4 mg)
- Questions
-
Additional Information found on packaging
TO INCREASE YOUR SUCCESS IN QUITTING:
- You must be motivated to quit.
- Use Enough - Chew at least 9 pieces of Nicotine Polacrilex Gum per day during the first six weeks.
- Use Long Enough - Use Nicotine Polacrilex Gum for the full 12 weeks.
- Use with a support program as directed in the enclosed User’s Guide.
To remove the gum, tear off single unit.
Peel off backing starting at corner with loose edge.
Push gum through foil.
Blister packaged for your protection.
Do not use if individual seals are open or torn.
- Not for sale to those under 18 years of age.
- Proof of age required.
- Not for sale in vending machines or from any source where proof of age cannot be verified.
Distributed by:
Sam's West, Inc.
Bentonville, AR 72716
DP22758
DP22759
-
Principal Display Panel
Compare to Nicorette® Fruit Chill™
Coated Gum active ingredient*
NDC 68196-015-01
Member's Mark™
2 mg
Coated Fruit Flavor
Sugar Free
Nicotine Gum
Nicotine Polacrilex Gum USP, (nicotine)
Stop Smoking Aid
FOR THOSE WHO SMOKE THEIR FIRST CIGARETTE
MORE THAN 30 MINUTES AFTER WAKING UPIf you smoke your first cigarette WITHIN 30 MINUTES
of waking up, use Nicotine Polacrilex Gum USP, 4 mgactual size
Coated Fruit Flavor231
Pieces*Nicorette® Fruit Chill™ are trademarks of GlaxoSmithKline Consumer Healthcare, L.P.
This product is not affiliated with GlaxoSmithKline Consumer Healthcare, L.P.DP22758

Nicotine Polacrilex Gum USP 2 mg Fruit Flavor
-
Principal Display Panel
Compare to Nicorette® Fruit Chill™
Coated Gum active ingredient*
NDC 68196-016-01
Member's Mark™
4 mg
Coated Fruit Flavor
Sugar Free
Nicotine Gum
Nicotine Polacrilex Gum USP, (nicotine)
Stop Smoking Aid
FOR THOSE WHO SMOKE THEIR FIRST CIGARETTE
WITHIN 30 MINUTES OF WAKING UPIf you smoke your first cigarette MORE THAN 30 MINUTES
after waking up, use Nicotine Polacrilex Gum USP, 2 mgactual size
Coated Fruit Flavor231
Pieces*Nicorette® Fruit Chill™ are trademarks of GlaxoSmithKline Consumer Healthcare, L.P.
This product is not affiliated with GlaxoSmithKline Consumer Healthcare, L.P.DP22759

Nicotine Polacrilex Gum USP 4 mg Fruit Flavor
- Principal Display Panel
- Principal Display Panel
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NICOTINE COATED FRUIT FLAVOR
nicotine polacrilex gum, chewingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68196-015 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NICOTINE (UNII: 6M3C89ZY6R) (NICOTINE - UNII:6M3C89ZY6R) NICOTINE 2 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) CARNAUBA WAX (UNII: R12CBM0EIZ) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYDROXYPROPYL CELLULOSE (90000 WAMW) (UNII: UKE75GEA7F) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM CARBONATE (UNII: 45P3261C7T) SUCRALOSE (UNII: 96K6UQ3ZD4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XYLITOL (UNII: VCQ006KQ1E) POLACRILIN (UNII: RCZ785HI7S) Product Characteristics Color brown (light brown) Score no score Shape SQUARE (biconvex) Size 14mm Flavor FRUIT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68196-015-01 231 in 1 CARTON 10/16/2017 1 33 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079044 10/16/2017 06/30/2021 NICOTINE COATED FRUIT FLAVOR
nicotine polacrilex gum, chewingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68196-016 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NICOTINE (UNII: 6M3C89ZY6R) (NICOTINE - UNII:6M3C89ZY6R) NICOTINE 4 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) CARNAUBA WAX (UNII: R12CBM0EIZ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYDROXYPROPYL CELLULOSE (90000 WAMW) (UNII: UKE75GEA7F) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM CARBONATE (UNII: 45P3261C7T) SUCRALOSE (UNII: 96K6UQ3ZD4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XYLITOL (UNII: VCQ006KQ1E) POLACRILIN (UNII: RCZ785HI7S) Product Characteristics Color white (light tan) Score no score Shape SQUARE (biconvex) Size 14mm Flavor FRUIT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68196-016-01 231 in 1 CARTON 10/16/2017 1 33 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079038 10/16/2017 Labeler - Sam's West, Inc. (051957769)