NITRONAL- nitroglycerin injection, solution
G. POHL-BOSKAMP GmbH & Co. KG
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Nitronal
Glyceryl trinitrate 1 mg/ml
2014-03-25
Dear Healthcare Professional,
Due to the current critical shortage of Nitroglycerin in 5% Dextrose Injection products in the United States (US) market, Arbor Pharmaceuticals, LLC (Arbor) is coordinating with the Food and Drug Administration (FDA) to increase the availability of Nitroglycerin in 5% Dextrose Injection products. In cooperation with the FDA, Arbor has initiated temporary importation of non-FDA approved NITRONAL (glyceryl trinitrate) Infusion solution, 1 mg/mL, from G. POHL BOSKAMP GmbH & Co. KG (Pohl-Boskamp), Germany into the US market.
Arbor will distribute NITRONAL (glyceryl trinitrate) Infusion solution 1 mg/ml. The concentration of nitroglycerin in NITRONAL is different from the concentration of nitroglycerin in the product typically available in the United States, but can be diluted to obtain concentrations similar to the FDA-approved product (see Table 1).
At this time, FDA is not objecting to the importation and distribution by Arbor of NITRONAL Infusion solution, 1 mg/mL made by Pohl-Boskamp, to address the critical shortage of Nitroglycerin in 5% Dextrose Injection. Importation or distribution of Pohl-Boskamp's NITRONAL Infusion solution, 1 mg/mL by any entity other than Arbor is not within the scope of this decision and may be subject to enforcement action by the FDA. FDA has not approved Pohl-Boskamp's NITRONAL Infusion solution, 1 mg/mL product in the United States. The product is marketed in Germany and other countries.
Effective immediately, Arbor will distribute the following presentations of NITRONAL Infusion solutions:
| 25 mL solution ampule containing 25 mg glyceryl trinitrate and 1225 mg glucose monohydrate (Dextrose monohydrate) | Authorization # 6008266.00.00 (Germany) 12 boxes of 10 ampules each (120 ampules per shipper carton) |
| 50 mL solution vial containing 50 mg glyceryl trinitrate and 2450 mg glucose monohydrate (Dextrose monohydrate) | Authorization # 6008266.00.00 (Germany) 8 bundles of 10 vials each (80 vials per shipper carton) |
The vial and carton labels will display the text used when marketing the products in English speaking countries. At the end of this letter you will find a product comparison table with the prescribing information in English, as well as images of the labels for your reference.
There are some key differences between the FDA-approved Nitroglycerin in 5% Dextrose Injection solution and Pohl-Boskamp's NITRONAL Infusion solution (glyceryl trinitrate), 1 mg/mL (please also see the product comparison tables attached).
-
Differences in nitroglycerin concentrations in these products are as follows:
- NITRONAL is available in 25 mg/25 mL ampules and in a 50 mg/50 mL vial, each providing a concentration of 1 mg/mL. This is a 2.5- to 10-fold higher nitroglycerin concentration than in FDA-approved Nitroglycerin in 5% Dextrose Injection solution.
- FDA-approved Nitroglycerin in 5% Dextrose Injection solution is provided in 250 mL glass containers at three concentrations of nitroglycerin: 25 mg/250 mL (100 mcg/mL), 50 mg/250 mL (200 mcg/mL) and 100 mg/250 mL (400 mcg/mL).
- NITRONAL can be added to 5% Dextrose Injection as described in Table 1 to obtain nitroglycerin concentrations of 25 mg/250 mL (100 mcg/mL), 50 mg/250 mL (200 mcg/mL) and 100 mg/250 mL (400 mcg/mL) like those for FDA-approved Nitroglycerin in 5% Dextrose Injection solution.
| Desired Nitroglycerin Concentration | Step 1: Start with 250 mL of D5W for Injection (in a container compatible with nitroglycerin) | Step 2: Volume of D5W Solution to Withdraw and Discard from 250 mL D5W for Injection Container | Step 3: Volume of NITRONAL*, 1 mg/mL nitroglycerin, to add to D5W for Injection Container | Resulting Nitroglycerin Concentration in 250 mL D5W for Injection |
|---|---|---|---|---|
| D5W = 5% Dextrose in water | ||||
|
||||
| 100 mcg/mL | 250 mL D5W for Injection | 25 mLs | 25 mL | 25 mg/250 mL of D5W, which is 100 mcg/mL |
| 200 mcg/mL | 250 mL D5W for Injection | 50 mLs | 50 mL | 50 mg/250 mL of D5W, which is 200 mcg/mL |
| 400 mcg/mL | 250 mL D5W for Injection | 100 mLs | 100 mL | 100 mg/250 mL of D5W, which is 400 mcg/mL |
- Table 2 provides flow rates for the 100, 200 and 400 mcg/mL concentrations of nitroglycerin in 5% Dextrose Injection (from Table 1) to obtain desired mcg/min doses.
Table 2. Necessary Flow Rates (mL/hr*) Desired Dose (mcg/min) Solution Concentration (mcg/mL) 100 200 400 - *
- With a set that produces 60 drops/mL, 1mL/hr = 1 drop/min.
5 3 1.5 0.8 10 6 3.0 1.5 15 9 4.5 2.3 20 12 6 3 30 18 9 4.5 40 24 12 6 50 30 15 7.5 60 36 18 9 80 48 24 12 100 60 30 15 120 72 36 18 140 84 42 21 160 96 48 24 180 108 54 27 200 120 60 30 240 144 72 36 280 168 84 42 320 192 96 48 500 300 150 75 - NITRONAL infusion solution contains glyceryl trinitrate as the active substance which is another name for the same active ingredient (nitroglycerin) in Nitroglycerin in Dextrose for Injection.
Nitroglycerin infusion solution is available only by prescription in the US.
Please refer to the package insert for the FDA-approved Nitroglycerin in 5% Dextrose Injection drug product for full prescribing information and follow the instructions presented in the FDA-approved package insert for safety recommendations.
The barcode may not register accurately on the U.S. scanning systems. Institutions should manually input the product into their systems and confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
If you have any questions about the information contained in this letter or the use of NITRONAL Infusion solution (glyceryl trinitrate), 1 mg/mL, please contact Arbor Pharmaceuticals, LLC at 1-866-516-4950 (Monday – Friday 9:00 am to 5:00 pm Eastern Daylight Time), or email medinfo@arborpharma.com.
To place an order, please contact Arbor Pharmaceuticals, LLC at 1-866-516-4950, extension 0 (Monday – Friday 9:00 am to 5:00 pm Eastern Daylight Time), or email GMB-SPS-ARBOR@cordlogistics.com.
The following products are available for order:
| NITRONAL (glyceryl trinitrate) Infusion solution; 1 mg/mL | NDC number | |
|---|---|---|
| 25 mL solution ampule (containing 25 mg glyceryl trinitrate and 1225 mg glucose monohydrate (Dextrose monohydrate)) | 12 boxes of 10 ampules each (120 ampules per shipper carton) | 62627-100-25 |
| 50 mL solution vial (containing 50 mg glyceryl trinitrate and 2450 mg glucose monohydrate (Dextrose monohydrate)) | 8 bundles of 10 vials each (80 vials per shipper carton) | 62627-100-50 |
We encourage healthcare providers to report any adverse events and medication errors that occur while using this product, as for any other drug. To report adverse events associated with NITRONAL Infusion solution (glyceryl trinitrate), 1 mg/mL, please call Arbor Pharmaceuticals, LLC at 1-866-516-4950 (Monday – Friday 9:00 am to 5:00 pm Eastern Daylight Time), or email aereport@arborpharma.com.
Adverse Events that may be related to the use of this product may also be reported to FDA's MedWatch Adverse Event Reporting Program either online, or regular mail or by fax:
- Online: www.fda.gov/medwatch/report.htm
- Regular mail: Use postage paid FDA form 3500 available at: www.fda.gov/medwatch/getforms.htm
Mail to MedWatch FDA, 5600 Fishers Lane, Rockville, MD 20852-0787 - Fax: 1-800-FDA-0178
We remain at your disposal to answer the questions you might have about our product, and provide more information if needed.
Sincerely,
POHL BOSKAMP
Marianne Boskamp
CEO
| Nitroglycerin in Dextrose (nitroglycerin) injection | NITRONAL(glyceryl trinitrate, 1 mg/mL) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition | ||||||||||||||||||||||||
| Active Ingredient | Nitroglycerin | Glyceryl trinitrate | ||||||||||||||||||||||
| Strength | 25 mg/250 mL (100 mcg/mL), 50 mg/250 mL (200 mcg/mL) and 100 mg/250 mL (400 mcg/mL) | 25 mg/25 mL (1 mg/mL) and 50 mg/50 mL (1 mg/mL) (only 25ml and 50ml sizes are shipped to the US) |
||||||||||||||||||||||
| Inactive Ingredients | Dextrose monohydrate, alcohol, citric acid monohydrate, sodium hydroxide, water, hydrochloric acid | Glucose monohydrate (Dextrose monohydrate), water, hydrochloric acid | ||||||||||||||||||||||
| Physiochemical Parameters | ||||||||||||||||||||||||
| pH | 4.0 (3.0 to 5.0) | 3.0-5.5 (not mentioned in the PIL) | ||||||||||||||||||||||
| Osmolarity (mOsmol/L (calc)) | 100 mcg/mL = 428 200 mcg/mL = 440 400 mcg/mL = 465 | 278 mOsmol/L, calculated, for the undiluted solution (not mentioned in the PIL) |
||||||||||||||||||||||
| Description | Sterile, nonpyrogenic solution of nitroglycerin and dextrose in water for injection. The solution is clear and practically colorless. Each 100 mL contains 10 mg, 20 mg, or 40 mg nitroglycerin (added as Diluted Nitroglycerin, USP with propylene glycol); 5 g Dextrose Hydrous, USP; 0.84 mL Alcohol, USP (added as a dissolution aid); and 105 mg Citric Acid Hydrous, USP (added as a buffer). The pH of the solution is adjusted with sodium hydroxide and, if necessary, hydrochloric acid. Although dry nitroglycerin is explosive, nitroglycerin in 5% dextrose is not. Indicated for treatment of peri-operative hypertension; for control of congestive heart failure in the setting of acute myocardial infarction; for treatment of angina pectoris in patients who have not responded to sublingual nitroglycerin and ß-blockers; and for induction of intraoperative hypotension. | Sterile, isotonic infusion solution indicated for angina pectoris, acute myocardial infarction, acute left ventricular failure, controlled hypotension, hypertensive crisis with cardiac decompensation, catheter-induced coronary spasms, and to increase ischaema tolerance during PTCA. | ||||||||||||||||||||||
| How Supplied | 250 mL glass containers | 25 mL ampoules and 50 mL vials | ||||||||||||||||||||||
| Country Specific Information | Marketing Authorization number in Germany: 6008266.00.00 USA: Special Import Permit, issued by the FDA |
|||||||||||||||||||||||
| Expiration date format | n/a | NITRONAL 25 mL ampules (closed container). NITRONAL 50 mL vials (closed container). Please see ampule or vial for expiration date. Format: MM/YYYY |
||||||||||||||||||||||
| Drug Status | Rx only | Rx only | ||||||||||||||||||||||
| Authorization holder | G. Pohl-Boskamp GmbH & Co. KG, 25551 Hohenlockstedt, Germany |
|||||||||||||||||||||||
| Comparison of prescribing information | ||||||||||||||||||||||||
| Clinical Pharmacology | The principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle and consequent dilatation of peripheral arteries and veins, especially the latter. Dilatation of the veins promotes peripheral pooling of blood and decreases venous return to the heart, thereby reducing left ventricular end-diastolic pressure and pulmonary capillary wedge pressure (preload). Arteriolar relaxation reduces systemic vascular resistance, systolic arterial pressure, and mean arterial pressure (afterload). Dilatation of the coronary arteries also occurs. The relative importance of preload reduction, afterload reduction, and coronary dilatation remains undefined. Dosing regimens for most chronically used drugs are designed to provide plasma concentrations that are continuously greater than a minimally effective concentration. This strategy is inappropriate for organic nitrates. Several well-controlled clinical trials have used exercise testing to assess the anti-anginal efficacy of continuously-delivered nitrates. In the large majority of these trials, active agents were indistinguishable from placebo after 24 hours (or less) of continuous therapy. Attempts to overcome nitrate tolerance by dose escalation, even to doses far in excess of those used acutely, have consistently failed. Only after nitrates have been absent from the body for several hours has their anti-anginal efficacy been restored. Pharmacokinetics: The volume of distribution of nitroglycerin is about 3 L/kg, and nitroglycerin is cleared from this volume at extremely rapid rates, with a resulting serum half-life of about 3 minutes. The observed clearance rates (close to 1 L/kg/min) greatly exceed hepatic blood flow; known sites of extrahepatic metabolism include red blood cells and vascular walls. The first products in the metabolism of nitroglycerin are inorganic nitrate and the 1,2-and 1,3- dinitroglycerols. The dinitrates are less effective vasodilators than nitroglycerin, but they are longer-lived in the serum, and their net contribution to the overall effect of chronic nitroglycerin regimens is not known. The dinitrates are further metabolized to (non-vasoactive) mononitrates and, ultimately, to glycerol and carbon dioxide. To avoid development of tolerance to nitroglycerin, drug-free intervals of 10-12 hours are known to be sufficient; shorter intervals have not been well studied. In one well-controlled clinical trial, subjects receiving nitroglycerin appeared to exhibit a rebound or withdrawal effect, so that their exercise tolerance at the end of the daily drug-free interval was less than that exhibited by the parallel group receiving placebo. Clinical Trials: Blinded, placebo-controlled trials of intravenous nitroglycerin have not been reported, but multiple investigators have reported open-label studies, and there are scattered reports of studies in which intravenous nitroglycerin was tested in blinded fashion against sodium nitroprusside. In each of these studies, therapeutic doses of intravenous nitroglycerin were found to reduce systolic and diastolic arterial blood pressure. The heart rate was usually increased, presumably as a reflexive response to the fall in blood pressure. Coronary perfusion pressure was usually, but not always, maintained. Intravenous nitroglycerin reduced central venous pressure (CVP), right atrial pressure (RAP), pulmonary arterial pressure (PAP), pulmonary-capillary wedge pressure (PCWP), pulmonary vascular resistance (PVR), and systemic vascular resistance (SVR). When these parameters were elevated, reducing them toward normal usually caused a rise in cardiac output. Conversely, intravenous nitroglycerin usually reduced cardiac output when it was given to patients whose CVP, RAP, PAP, PCWP, PVR, and SVR were all normal. Most clinical trials of intravenous nitroglycerin have been brief; they have typically followed hemodynamic parameters during a single surgical procedure. In one careful study, one of the few that lasted more than a few hours, continuous intravenous nitroglycerin had lost almost all of its hemodynamic effect after 48 hours. In the same study, patients who received nitroglycerin infusions for only 12 hours out of each 24 demonstrated no similar attenuation of effect. These results are consistent with those seen in multiple large, double-blind, placebo-controlled trials of other formulations of nitroglycerin and other nitrates. | Pharmacological effect: Glyceryl trinitrate has a direct relaxing effect on smooth vascular muscles and causes a vasodilatation. |
||||||||||||||||||||||
| Indications and Usage | Nitroglycerin in 5% Dextrose Injection is indicated for treatment of peri-operative hypertension; for control of congestive heart failure in the setting of acute myocardial infarction; for treatment of angina pectoris in patients who have not responded to sublingual nitroglycerin and ß-blockers; and for induction of intraoperative hypotension. |
|
||||||||||||||||||||||
| Contraindications | Allergic reactions to organic nitrates are extremely rare, but they do occur. Nitroglycerin in 5% Dextrose Injection is contraindicated in patients who are allergic to it. In patients with pericardial tamponade, restrictive cardiomyopathy, or constrictive pericarditis, cardiac output is dependent upon venous return. Intravenous nitroglycerin is contraindicated in patients with these conditions. Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products. | Glyceryl trinitrate must not be used in patients with:
Glyceryl trinitrate may only be administered carefully with:
In volume depleted patients, adequate volume replacement is required at the start of treatment. |
||||||||||||||||||||||
| Warnings Amplification of the vasodilatory effects of Nitroglycerin by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied. Appropriate supportive care has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion. Nitroglycerin readily migrates into many plastics, including the polyvinyl chloride (PVC) plastics commonly used for intravenous administration sets. Nitroglycerin absorption by PVC tubing is increased when the tubing is long, the flow rates are low, and the nitroglycerin concentration of the solution is high. The delivered fraction of the solution's original nitroglycerin content has been 20-60% in published studies using PVC tubing; the fraction varies with time during a single infusion, and no simple correction factor can be used. PVC tubing has been used in most published studies of intravenous nitroglycerin, but the reported doses have been calculated by simply multiplying the flow rate of the solution by the solution's original concentration of nitroglycerin. The actual doses delivered have been less, sometimes much less, than those reported. Relatively non-absorptive intravenous administration sets are available. If intravenous nitroglycerin is administered through non-absorptive tubing, doses based upon published reports will generally be too high. Some in-line intravenous filters also absorb nitroglycerin; these filters should be avoided. Solutions containing dextrose without electrolytes should not be administered through the same administration set as blood, as this may result in pseudoagglutination or hemolysis. The intravenous administration of solutions may cause fluid overloading resulting in dilution of serum electrolyte concentrations, overhydration and congested states of pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentrations of the injections. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentration of the injections. Precautions General: Severe hypotension and shock may occur with even small doses of nitroglycerin. This drug should therefore be used with caution in patients who may be volume depleted or who, for whatever reason, are already hypotensive. Hypotension induced by nitroglycerin may be accompanied by paradoxical bradycardia and increased angina pectoris. Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy. As tolerance to other forms of nitroglycerin develops, the effect of sublingual nitroglycerin on exercise tolerance, although still observable, is somewhat blunted. In industrial workers who have long-term exposure to unknown (presumably high) doses of organic nitrates, tolerance clearly occurs. Chest pain, acute myocardial infarction, and even sudden death have occurred during temporary withdrawal of nitrates from these workers, demonstrating the existence of true physical dependence. Some clinical trials in angina patients have provided nitroglycerin for about 12 continuous hours of every 24-hour day. During the nitrate-free intervals in some of these trials, anginal attacks have been more easily provoked than before treatment, and patients have demonstrated hemodynamic rebound and decreased exercise tolerance. The importance of these observations to the routine, clinical use of intravenous nitroglycerin is not known. Lower concentrations of Nitroglycerin in 5% Dextrose Injection increase the potential precision of dosing, but these concentrations increase the total fluid volume that must be delivered to the patient. Total fluid load may be a dominant consideration in patients with compromised function of the heart, liver, and/or kidneys. Nitroglycerin in 5% Dextrose Injection should be administered only via an infusion pump that can maintain a constant infusion rate. Intracoronary injection of Nitroglycerin in 5% Dextrose Injection has not been studied. Solutions containing dextrose should be used with caution in patients with known sub-clinical or overt diabetes mellitus. Laboratory Tests: Because of the propylene glycol content of intravenous nitroglycerin, serum triglyceride assays that rely on glycerol oxidase may give falsely elevated results in patients receiving this medication. Drug Interactions: The vasodilating effects of nitroglycerin may be additive with those of other vasodilators. Marked symptomatic orthostatic hypotension has been reported when calcium channel blockers and organic nitrates were used in combination. Intravenous nitroglycerin interferes, at least in some patients, with the anticoagulant effect of heparin. In patients receiving intravenous nitroglycerin, concomitant heparin therapy should be guided by frequent measurement of the activated partial thromboplastin time. Administration of Nitroglycerin in 5% Dextrose Injection through the same infusion set as blood can result in pseudoagglutination and hemolysis. More generally, Nitroglycerin in 5% Dextrose Injection should not be mixed with any other medication of any kind. Carcinogenesis, Mutagenesis, Impairment of Fertility: Animal carcinogenesis studies with injectable nitroglycerin have not been performed. Rats receiving up to 434 mg/kg/day of dietary nitroglycerin for 2 years developed dose-related fibrotic and neoplastic changes in liver, including carcinomas, and interstitial cell tumors in testes. At high dose, the incidences of hepatocellular carcinomas in both sexes were 52% vs. 0% in controls and incidences of testicular tumors were 52% vs. 8% in controls. Lifetime dietary administration of up to 1058 mg/kg/day of nitroglycerin was not tumorigenic in mice. Nitroglycerin was weakly mutagenic in Ames tests performed in two different laboratories. Nevertheless, there was no evidence of mutagenicity in an in vivo dominant lethal assay with male rats treated with doses up to about 363 mg/kg/day, p.o., or in in vitro cytogenetic tests in rat and dog tissues. In a three-generation reproduction study, rats received dietary nitroglycerin at doses up to about 434 mg/kg/day for six months prior to mating of the F0 generation with treatment continuing through successive F1 and F2 generations. The high-dose was associated with decreased feed intake and body weight gain in both sexes at all matings. No specific effect on the fertility of the F0 generation was seen. Infertility noted in subsequent generations, however, was attributed to increased interstitial cell tissue and aspermatogenesis in the high-dose males. In this three-generation study there was no clear evidence of teratogenicity. Pregnancy Category C: Animal teratology studies have not been conducted with nitroglycerin injection. Teratology studies in rats and rabbits were conducted with topically applied nitroglycerin ointment at doses up to 80 mg/kg/day and 240 mg/kg/day, respectively, and no toxic effects on dams or fetuses were seen. There are no adequate and well-controlled studies in pregnant women. Nitroglycerin should be given to a pregnant woman only if clearly needed. Nursing Mothers: It is not known whether nitroglycerin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when nitroglycerin is administered to a nursing woman. Pediatric Use: Safety and effectiveness in the pediatric population have not been established. However, the relationship between hemodynamic effects of nitroglycerin and dose in the pediatric population have been documented in the literature. | As an extra precaution, glyceryl trinitrate may only be taken during pregnancy and lactation, if specifically directed by a doctor, since there is not enough experience with pregnant and nursing women. Experiments in animals have yielded no evidence of damage to the fetus. | |||||||||||||||||||||||
| Table 2. | ||||||||||||||||||||||||
| Studies in the literature used doses of nitroglycerin injection in pediatric patients ranging from 0.5 to 5 mcg/kg/min. The following chart can be used to calculate the flow rate in mL/hour of nitroglycerin using the 100 mcg/mL (25 mg/250 mL) concentration of nitroglycerin. Note: Very low infusion rates may require that a more dilute concentration of nitroglycerin infusion solution be prepared. | ||||||||||||||||||||||||
| Flow Rate (mL/hour) Using the 100 mcg/mL (25mg/250 mL) Concentration | ||||||||||||||||||||||||
| Dose (mcg/ kg/ min) | Patient Weight in kg | |||||||||||||||||||||||
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | |||||||||||||||
| 0.1* | 0.12 | 0.24 | 0.36 | 0.48 | 0.6 | 0.72 | 0.84 | 0.96 | 1.08 | 1.2 | ||||||||||||||
| 0.5 | 0.6 | 1.2 | 1.8 | 2.4 | 3 | 3.6 | 4.2 | 4.8 | 5.4 | 6 | ||||||||||||||
| 1 | 1.2 | 2.4 | 3.6 | 4.8 | 6 | 7.2 | 8.4 | 9.6 | 10.8 | 12 | ||||||||||||||
| 2 | 2.4 | 4.8 | 7.2 | 9.6 | 12 | 14.4 | 16.8 | 19.2 | 21.6 | 24 | ||||||||||||||
| 3 | 3.6 | 7.2 | 10.8 | 14.4 | 18 | 21.6 | 25.2 | 28.8 | 32.4 | 36 | ||||||||||||||
| 4 | 4.8 | 9.6 | 14.4 | 19.2 | 24 | 28.8 | 33.2 | 38.4 | 43.2 | 48 | ||||||||||||||
| 5 | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | ||||||||||||||
| 10* | 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 | 108 | 120 | ||||||||||||||
| * Dose not studied in pediatric trials | ||||||||||||||||||||||||
|
Geriatric Use: Clinical studies of Nitroglycerin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Do not use unless vacuum is present and solution is clear. | ||||||||||||||||||||||||
| Adverse Reactions | Adverse reactions to nitroglycerin are generally dose-related and almost all of these reactions are the result of nitroglycerin's activity as a vasodilator. Headache, which may be severe, is the most commonly reported side effect. Headache may be recurrent with each daily dose, especially at higher doses. Transient episodes of lightheadedness, occasionally related to blood pressure changes, may also occur. Hypotension occurs infrequently, but in some patients it may be severe enough to warrant discontinuation of therapy. Syncope, crescendo angina, and rebound hypertension have been reported but are uncommon. Allergic reactions to nitroglycerin are also uncommon, and the great majority of those reported have been cases of contact dermatitis or fixed drug eruptions in patients receiving nitroglycerin in ointments or patches. There have been a few reports of genuine anaphylactoid reactions, and these reactions can probably occur in patients receiving nitroglycerin by any route. Extremely rarely, ordinary doses of organic nitrates have caused methemoglobinemia in normal-seeming patients. Methemoglobinemia is so infrequent at these doses that further discussion of its diagnosis and treatment is deferred (see Overdosage). Data are not available to allow estimation of the frequency of adverse reactions during treatment with Nitroglycerin in 5% Dextrose Injection. | At the start of therapy, a nitrate-induced headache can frequently occur, but usually subsides with continued use. A dose-dependent drop in blood pressure and a heart rate increase may occur. In case of a more severe drop in blood pressure, the infusion must be discontinued. If no spontaneous recovery follows, possibly cardio vascular measures have to be taken, e. g. elevation of the legs or volume replacement. Rare events, which may occur: nausea, vomiting, transient redness of the skin (flush) and allergic skin reactions. In rare cases, with a large drop in blood pressure angina pectoris symptoms may be intensified (paradoxical nitrate reaction). Rare events, which can be observed: collapse states, occasionally with cardiac dysrhythmia and a slower pulse rate (bradicardial arrhythmia) and syncope (sudden loss of consciousness). In individual cases exfoliative dermatitis (inflammatory skin disease) may occur. Tolerance development and the occurrence of cross tolerance to other nitro compounds have been described. In order to avoid an attenuation or loss of effect, high continuous dosage should be avoided. Note: During the infusion of NITRONAL, a transient hypoxaemia may occur due to a relative redistribution of the blood flow in hypoventilated alveolar regions, and in patients with coronary heart disease it may lead to ischaemia. Even when used as directed, this drug may affect the ability to drive or operate machinery. This can occur in particular at the beginning of the treatment, with an increase of the dosage, when changing the medicinal product, or when used in combination with alcohol. |
||||||||||||||||||||||
| Drug abuse and dependence | None known | Tolerance development and the occurrence of cross tolerance to other nitro compounds have been described. In order to avoid an attenuation or loss of effect, high continuous dosage should be avoided. (See side effects) | ||||||||||||||||||||||
| Hemodynamic Effects:
The ill effects of nitroglycerin overdose are generally the results of nitroglycerin's capacity to induce vasodilation, venous pooling, reduced cardiac output, and hypotension. These hemodynamic changes may have protean manifestations, including increased intracranial pressure, with any or all of persistent throbbing headache, confusion, and moderate fever; vertigo; palpitations; visual disturbances; nausea and vomiting (possibly with colic and even bloody diarrhea); syncope (especially in the upright posture); air hunger and dyspnea, later followed by reduced ventilatory effort; diaphoresis, with the skin either flushed or cold and clammy; heart block and bradycardia; paralysis; coma; seizures; and death. Laboratory determinations of serum levels of nitroglycerin and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of nitroglycerin overdose. No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of nitroglycerin and its active metabolites. Similarly, it is not known which -if any-of these substances can usefully be removed from the body by hemodialysis. No specific antagonist to the vasodilator effects of nitroglycerin is known, and no intervention has been subject to controlled study as a therapy of nitroglycerin overdose. Because the hypotension associated with nitroglycerin overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. Passive elevation of the patient's legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary. The use of epinephrine or other arterial vasoconstrictors in this setting is likely to do more harm than good. In patients with renal disease or congestive heart failure, therapy resulting in central volume expansion is not without hazard. Treatment of nitroglycerin overdose in these patients may be subtle and difficult, and invasive monitoring may be required. Methemoglobinemia: Nitrate ions liberated during metabolism of nitroglycerin can oxidize hemoglobin into methemoglobin. Even in patients totally without cytochrome b5 reductase activity, however, and even assuming that the nitrate moieties of nitroglycerin are quantitatively applied to oxidation of hemoglobin, about 1 mg/kg of nitroglycerin should be required before any of these patients manifests clinically significant (≥ 10%) methemoglobinemia. In patients with normal reductase function, significant production of methemoglobin should require even larger doses of nitroglycerin. In one study in which 36 patients received 2-4 weeks of continuous nitroglycerin therapy at 3.1 to 4.4 mg/hr, the average methemoglobin level measured was 0.2%; this was comparable to that observed in parallel patients who received placebo. Notwithstanding these observations, there are case reports of significant methemoglobinemia in association with moderate overdoses of organic nitrates. None of the affected patients had been thought to be unusually susceptible. Methemoglobin levels are available from most clinical laboratories. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate cardiac output and adequate arterial pO2. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air. When methemoglobinemia is diagnosed, the treatment of choice is methylene blue, 1-2 mg/kg intravenously. | a) Symptoms of overdose Drop in blood pressure with orthostatic regulatory disturbances, reflex tachycardia and headaches, weakness, dizziness, somnolence, flush, nausea, vomiting and diarrhoea may occur. At high doses (more than 20 mg / kg body-weight) methaemoglobinemia, cyanosis, dyspnoea and tachypnea must be anticipated owing to nitrite ions formed during the metabolism of glyceryl trinitrate. At very high doses an increase in intracranial pressure with cerebral symptoms may occur. At chronic overdosage increased methaemoglobin levels were measured of which the clinical relevance is controversial. b) Treatment in the event of overdose In addition to general emergency procedures such as gastric lavage and placing the patient in the recumbent position with the legs raised, the vital parameters must be monitored under intensive-care conditions and corrected if required. If there is pronounced hypotension and/or shock a volume replacement should be performed; in exceptional cases, norepinephrine and / or dopamine can be infused as a cardiovascular therapy. Administration of epinephrine and related substances is contra-indicated. If methaemoglobinemia occurs, the following antidotes can be used depending on the degree of severity: 1. Vitamin C: 1 g PO or as the sodium salt IV 2. Methylene blue: up to 50 mL of a 1 % methylene blue solution IV 3. Toluidine blue: initial dose 2 – 4 mg / kg body-weight, IV only; if necessary, repeated dose of 2 mg / kg body-weight in intervals of 1hour. 4. Oxygen therapy, haemodialysis, exchange transfusion. |
|||||||||||||||||||||||
| Dosage and Administration | Nitroglycerin in 5% Dextrose Injection is intended for intravenous administration using sterile equipment. It should be administered only via an infusion pump that can maintain a constant infusion rate. A container which has lost its vacuum, or one in which particulate matter is visible, should not be used. Dosage is affected by the type of infusion set used (see Warnings). Although the usual adult starting dose in published studies has been 25 mcg/min or more, these studies used PVC tubing, so the delivered doses were less than those reported. When nonabsorptive tubing is used, doses must be reduced. Even using nonabsorptive tubing, the dose necessary to achieve a given response will vary greatly from patient to patient. Patients with normal or low left-ventricular filling pressure (e.g., patients with uncomplicated angina pectoris) may respond fully to as little as 5 mcg/min, while other patients may require a dose that is one or even two orders of magnitude higher. Continuous monitoring of blood pressure and heart rate is necessary in all patients receiving this medication; in many cases, invasive monitoring of pulmonary capillary wedge pressure will also be indicated. Lower concentrations of Nitroglycerin in 5% Dextrose Injection increase the potential precision of dosing, but these concentrations increase the total fluid volume that must be delivered to the patient. Total fluid load may be a dominant consideration in patients with compromised function of the heart, liver, and/or kidneys. The necessary flow rates to achieve various dose rates with the available concentrations are shown in the following table. Using nonabsorptive tubing, the initial adult dosage of Nitroglycerin in 5% Dextrose Injection should be 5 mcg/min. Subsequent titration must be guided by the clinical results, with dose increments becoming more cautious as partial response is seen. Initial titration should be in 5 mcg/min increments at intervals of 3 to 5 minutes. If no response is seen at 20 mcg/min, increments of 10 and even 20 mcg/min can be used. Once some hemodynamic response is observed, dosage increments should be smaller and less frequent. When the concentration is changed, the tubing must be disconnected from the patient and flushed with the new solution before therapy is continued. If this precaution is not taken, then depending upon the tubing, pump, and flow rate used, it might be several hours before nitroglycerin is delivered at the desired rate. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not add supplementary medication to Nitroglycerin in 5% Dextrose Injection. | (Please also see Dosage and flow rate information in the Dear Healthcare Professional letter) Depending on the initial clinical and haemodynamic values, the dosage is determined by the patient's individual requirement and the response of the parameters to be controlled. For clinical use, start with a dose of 0.5 –1.0 mg glyceryl trinitrate per hour and adjust the dosage to meet individual requirements; the maximum dose is as a rule 8 mg glyceryl trinitrate per hour, rarely 10 mg per hour. In acute myocardial infarction, continuous intravenous infusion should be started as soon as possible. If the systolic pressure exceeds 100 mm Hg, 2 – 8 mg may be infused per hour (33 –133 mcg per minute), in exceptional cases up to 10 mg per hour (166 mcg per minute) until the symptoms of angina pectoris subside. In acute left ventricular failure (pulmonary oedema): 2 – 8 mg per hour (33 –133 mcg per minute), for 1– 2 days In severe angina pectoris, the patient should be placed in intensive care and treated with a dose of 2 to 8 mg per hour (33 –133 mcg per minute). The haemodynamic status must be checked continuously during the infusion. Constant monitoring of systolic and diastolic blood pressure, heart rate and haemodynamic parameters (right heart catheter) such as pulmonary arterial systolic pressure (PASP), pulmonary capillary pressure (PCP), pulmonary arterial diastolic pressure (PADP), cardiac output (CO) and ECG (measurement of the ST segment) is also necessary. In hypertonic crisis with cardiac decompensation, infuse with continuous monitoring of blood pressure and heart rate at 2 – 8 mg per hour (average 5 mg per hour). For controlled hypotension, depending on the anaesthetic procedure and the desired blood pressure reduction, 2 –10 mcg per kg body-weight per minute under ECG control and invasive blood pressure control. In patients with impaired hepatic and renal function, the dose should be reduced according to the severity of the dysfunction. In order to avoid reduction or loss of activity, select the lowest possible clinically effective dose; if appropriate, intermittent administration or alternating treatment with other vasodilators should be considered. Method and duration of use: The intravenous infusion of glyceryl trinitrate should take place in a hospital setting and under continuous Cardiovascular control. NITRONAL can be infused either undiluted using appropriate devices or diluted (e. g. with physiolo gical saline solution, glucose 5 %). When using the drug in combination with other infusion solutions, the manufacturer's information on the solution concerned must be observed, including compatibility, contraindications, adverse effects and drug interactions. |
||||||||||||||||||||||
| Dilution table | ||||||||||||||||||||||||
| Table 3 Necessary Flow Rates (mL/hr*) | Amount of active ingredient (glyceryl trinitrate) | 5 mg | 10 mg | 20 mg | 30 mg | 40 mg | 50 mg | |||||||||||||||||
| Desired Dose (mcg/min) | Solution Concentration (mcg/mL) | |||||||||||||||||||||||
| 100 | 200 | 400 | NITRONAL aqueous solution | 5 ml | 10 ml | 20 ml | 30 ml | 40 ml | 50 ml | |||||||||||||||
| 5 | 3 | 1.5 | 0.8 | |||||||||||||||||||||
| 10 | 6 | 3.0 | 1.5 | Infusion solution on dilution | 1 + 10 1 + 20 1 + 40 | 50 ml 100 ml 200 ml | 100 ml 200 ml 400 ml | 200 ml 400 ml 800 ml | 300 ml 600 ml 1200 ml | 400 ml 800 ml 1600 ml | 500 ml 1000 ml 2000 ml | |||||||||||||
| 15 | 9 | 4.5 | 2.3 | |||||||||||||||||||||
| 20 | 12 | 6 | 3 | |||||||||||||||||||||
| 30 | 18 | 9 | 4.5 | |||||||||||||||||||||
| 40 | 24 | 12 | 6 | Finished solution | 1 + 10 1 + 20 1 + 40 | 55 ml 105 ml 205 ml | 110 ml 210 ml 410 ml | 220 ml 420 ml 820 ml | 330 ml 630 ml 1230 ml | 440 ml 840 ml 1640 ml | 550 ml 1050 ml 2050 ml | |||||||||||||
| 50 | 30 | 15 | 7.5 | |||||||||||||||||||||
| 60 | 36 | 18 | 9 | |||||||||||||||||||||
| 80 | 48 | 24 | 12 | |||||||||||||||||||||
| 100 | 60 | 30 | 15 | |||||||||||||||||||||
| 120 | 72 | 36 | 18 | Infusion table | ||||||||||||||||||||
| 140 | 84 | 42 | 21 | Dilution | 1 + 10 | 1 + 20 | 1 + 40 | |||||||||||||||||
| 160 | 96 | 48 | 24 | Desired glyceryl trinitrate amount/h | INFUSION | |||||||||||||||||||
| 180 | 108 | 54 | 27 | |||||||||||||||||||||
| 200 | 120 | 60 | 30 | ml/h | drops/min | ml/h | drops/min | ml/h | drops/min | |||||||||||||||
| 240 | 144 | 72 | 36 | 0.50 mg | 5.5 | 2 | 10.5 | 3 - 4 | 20.5 | 6 - 7 | ||||||||||||||
| 280 | 168 | 84 | 42 | 0.75 mg | 8.25 | 3 | 15.75 | 5 | 30.75 | 10 | ||||||||||||||
| 320 | 192 | 96 | 48 | 1.0 mg | 11.0 | 3 - 4 | 21.0 | 7 | 41.0 | 13 - 14 | ||||||||||||||
| 500 | 300 | 150 | 75 | 1.25 mg | 13.75 | 4 - 5 | 26.25 | 8 - 9 | 51.25 | 17 | ||||||||||||||
| * With a set that produces 60 drops/mL, 1mL/hr = 1 drop/min. | 1.5 mg | 16.5 | 5 - 6 | 31.5 | 10 - 11 | 61.5 | 20 - 21 | |||||||||||||||||
| 2.0 mg | 22.0 | 6 - 7 | 42.0 | 14 | 82.0 | 26 - 27 | ||||||||||||||||||
| 2.5 mg | 27.5 | 9 | 52.5 | 17 | 102.5 | 34 | ||||||||||||||||||
| 3.0 mg | 33.0 | 11 | 63.0 | 21 | 123.0 | 41 | ||||||||||||||||||
| 3.5 mg | 38.5 | 12 - 13 | 73.5 | 24 - 25 | 143.5 | 47 - 48 | ||||||||||||||||||
| 4.0 mg | 44.0 | 13 | 84.0 | 28 | 164.0 | 53 | ||||||||||||||||||
| 4.5 mg | 49.5 | 14 - 15 | 94.5 | 31 - 32 | 184.5 | 59 - 60 | ||||||||||||||||||
| 5.0 mg | 55.0 | 18 | 105.0 | 35 | 205.0 | 68 | ||||||||||||||||||
| 5.5 mg | 60.5 | 20 | 115.5 | 38 - 39 | 225.5 | 74 - 75 | ||||||||||||||||||
| 6.0 mg | 66.0 | 22 | 126.0 | 42 | 246.0 | 82 | ||||||||||||||||||
| 7.0 mg | 77.0 | 25 - 26 | 147.0 | 49 | 287.0 | 95 - 96 | ||||||||||||||||||
| 8.0 mg | 88.0 | 28 - 29 | 168.0 | 56 | 328.0 | 108 - 109 | ||||||||||||||||||
| 9.0 mg | 99.0 | 31 - 32 | 189.0 | 63 | 369.0 | 121 - 122 | ||||||||||||||||||
| 10.0 mg | 110.0 | 36 | 210.0 | 70 | ||||||||||||||||||||
| Depending on clinical status, haemodynamics and ECG, treatment may be continued for up to three days or longer. The medical practitioner decides on the duration of use. Note: For the infusion of NITRONAL polyethylene or polytetrafluorethylene tubings proved worthwhile. Polyvinylchloride tubings lead to a considerable loss of active substance due to adsorption. |
||||||||||||||||||||||||
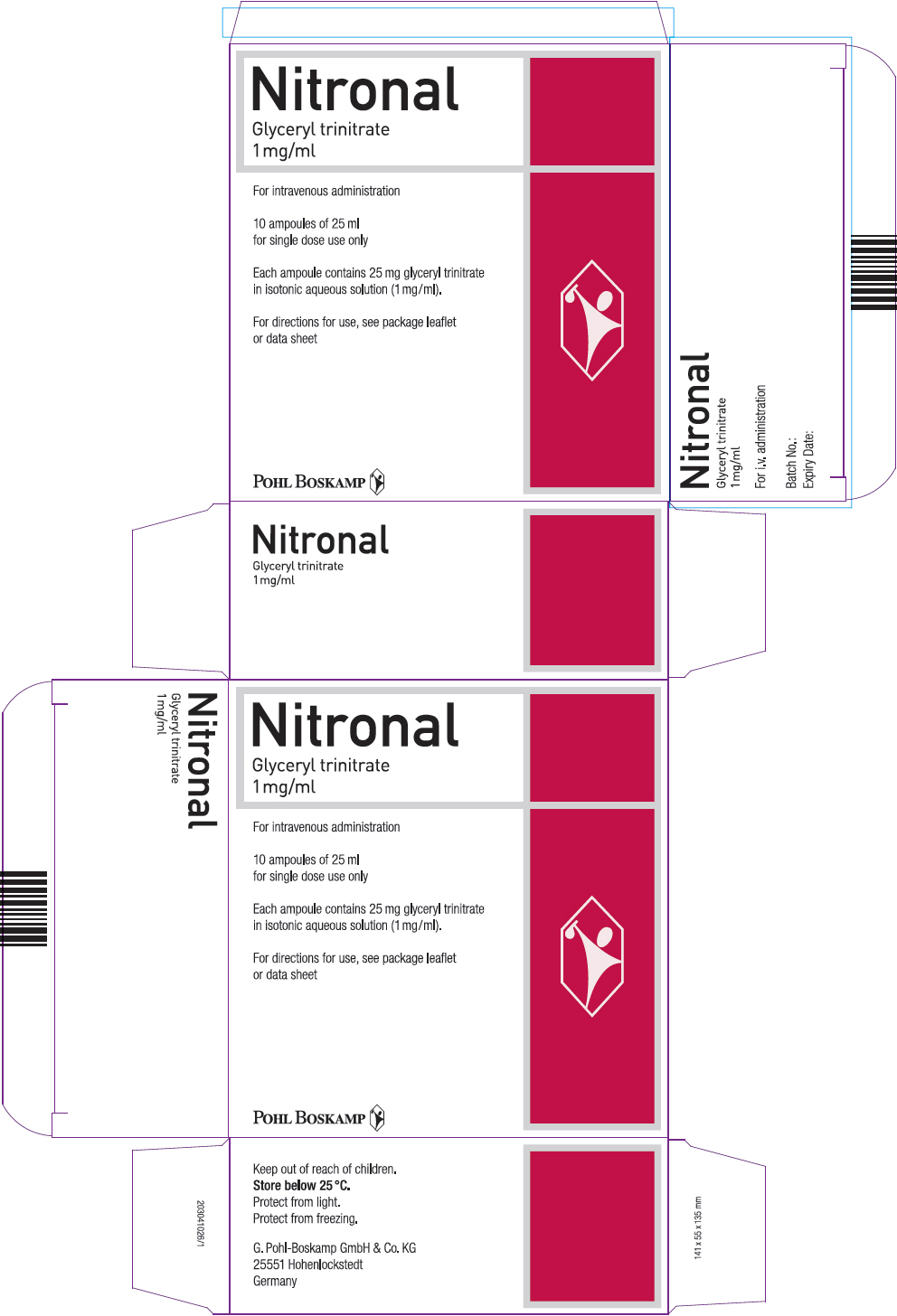
PRINCIPAL DISPLAY PANEL - 25 ml Ampule Carton
Nitronal
Glyceryl trinitrate
1 mg/ml
For intravenous administration
10 ampoules of 25 ml
for single dose use only
Each ampoule contains 25 mg glyceryl trinitrate
in isotonic aqueous solution (1 mg/ml).
For directions for use, see package leaflet
or data sheet
POHL BOSKAMP
| NITRONAL
nitroglycerin injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - G. POHL-BOSKAMP GmbH & Co. KG (315881672) |