DISCUS COMPOSITUM RX - pseudognaphalium obtusifolium, berberis vulgaris root bark, black cohosh, cinchona officinalis bark, citrullus colocynthis fruit pulp, ledum palustre twig, ranunculus bulbosus, horse chestnut, ascorbic acid, cupric acetate, potassium carbonate, nadide, sodium diethyl oxalacetate, niacinamide, trinitrophenol, pulsatilla vulgaris, pyridoxine hydrochloride, riboflavin, claviceps purpurea sclerotium, silicon dioxide, thiamine hydrochloride, ammonium chloride, sus scrofa cartilage, sus scrofa intervertebral disc, .alpha.-lipoic acid, silver, tribasic calcium phosphate, coenzyme a, sus scrofa embryo, sus scrofa umbilical cord, sus scrofa adrenal gland, sus scrofa bone marrow, mercuric oxide, yellow, sepia officinalis juice, zinc, gonorrheal urethral secretion human and sulfur tablet
Heel Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
DESCRIPTION
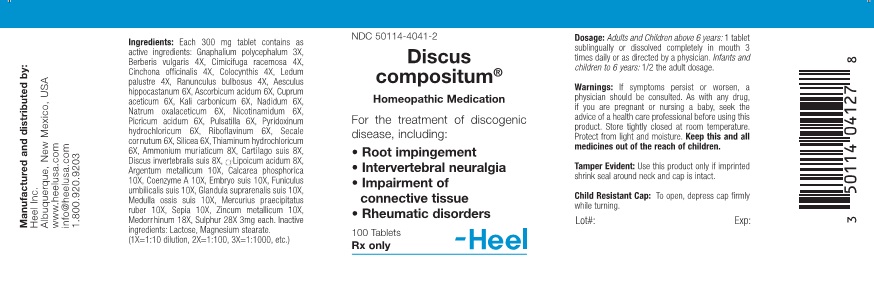
Each 300 mg tablet contains as active ingredients: Gnaphalium polycephalum 3X, Berberis vulgaris 4X, Cimicifuga racemosa 4X, Cinchona officinalis 4X, Colocynthis 4X, Ledum palustre 4X, Ranunculus bulbosus 4X, Aesculus hippocastanum 6X, Ascorbicum acidum 6X, Cuprum aceticum 6X, Kali carbonicum 6X, Nadidum 6X, Natrum oxalaceticum 6X, Nicotinamidum 6X, Picricum acidum 6X, Pulsatilla 6X, Pyridoxinum hydrochloricum 6X, Riboflavinum 6X, Secale cornutum 6X Silicea 6X, Thiaminum hydrochloricum 6X, Ammonium muriaticum 8X, Cartilago suis 8X, Discus invertebralis suis 8X, a-Lipoicum acidum 8X, Argentum metallicum 10X, Calcarea phosphorica 10X, Coenzyme A 10X, Embryo suis 10X, Funiculus umbilicalis suis 10X, Glandula suprarenalis suis 10X, Medulla ossis suis 10X, Mercurius praecipitatus ruber 10X, Sepia 10X, Zincum metallicum 10X, Medorrhinum 18X, Sulphur 28X 3mg each. Inactive ingredients: Lactose, Magnesium stearate.
INDICATION AND USAGE
For the treatment of discogenic disease including:
- Root impingement
- Intervertebral neuralgia
- Impairment of connective tissue
- Rheumatic dosorders
DOSAGE AND ADMINISTRATION
Adults and children above 6 years: 1 tablet sublingually or dissolved completely in mouth 3 times daily or as directed by a physician. Infants and children to 6 years: 1/2 the adult dosage.
WARNINGS
If symptoms persist or worsen, a physician should be consulted. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health care professional before using this product. Keep this and all medicines out of the reach of children.