COLD MEDICINE XL-3 FOR CHILDREN- acetaminophen chlorpheniramine maleate phenylepherine hydrochloride tablet, chewable
Selder, S.A. de C.V.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
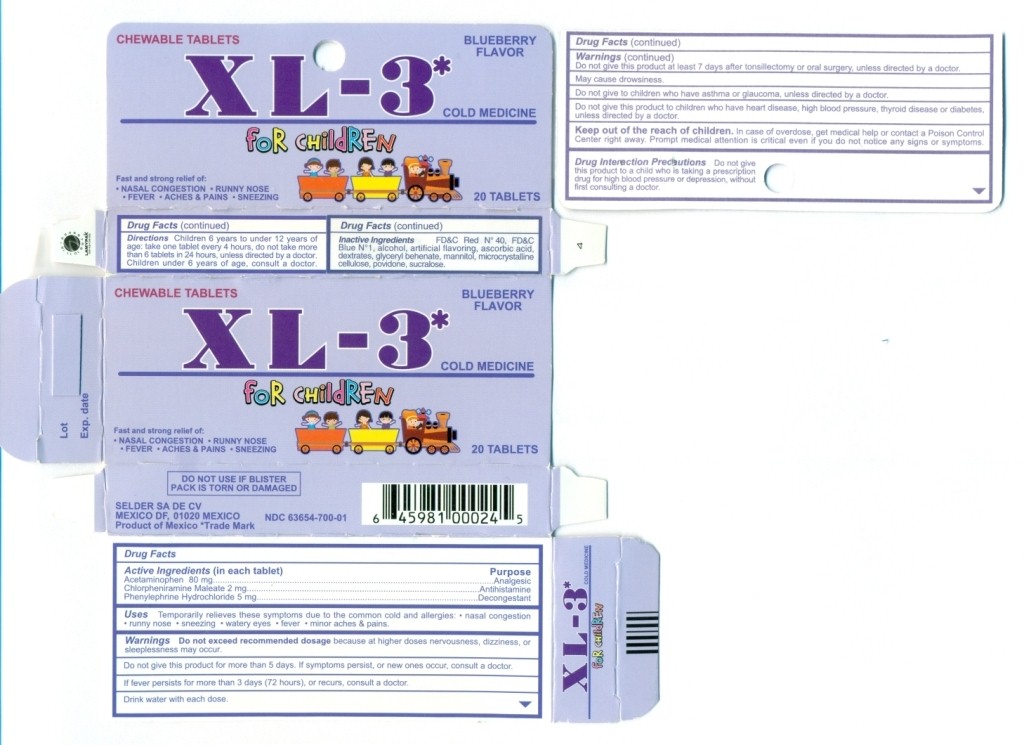
Drug Facts
Active Ingredients (in each tablet)
Acetaminophen 80 mg
Chlorpheniramine Maleate 2 mg
Phenylepherine Hydrochloride 5 mg
Uses
temporarily relieves these symptoms due to common cold and allergies: • cough • nasal congestion • runny nose • sneezing • watery eyes • fever • aches and pains
Warnings
Do not exceed recommended dosage because at higher dosages nervousness, dizziness, or sleeplessness may occur. Do not give the product for more than 5 days.
If symptoms persist, or if new ones occur consult a doctor.
If fever persists for more than 3 days (72 hours), or recurs, consult a doctor.
Drink water with each dose
Do not give this product at least after 7 days after a tonsillectomy or oral surgery, unless directed by a doctor.
May cause drowsiness
Do not give to children who have asthma or glaucoma, unless directed by a doctor.
Do not give this product to children who have heart disease, high blood pressure, thyroid disease or diabetes, unless directed by a doctor.
Keep this and all drugs out of the reach of children
In case of accidental overdose, get medical help or contact a Poison Control Center immediately. Prompt medical attention is critical for adults as well as children even if you do not notice any signs or symptoms.
Drug Interaction Precautions
Do not give this product to a child who is taking a prescription drug for high blood pressure or depression, without first consulting a doctor
Directions
Children 6 years of age to under 12 years: take 1 tablet every 4 hours, do not take more than 6 tablets in 24 hours, unless directed by a doctor. Children under 6 years of age, consult a doctor
| COLD MEDICINE
XL-3 FOR CHILDREN
acetaminophen chlorpheniramine maleate phenylepherine hydrochloride tablet, chewable |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Selder, S.A. de C.V. (824413629) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Selder, S.A. de C.V. | 824413629 | manufacture(63654-700) | |