IZBA - travoprost solution
Alcon Laboratories, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use IZBA safely and effectively. See full prescribing information for IZBA.
IZBA® (travoprost ophthalmic solution) 0.003%, for topical ophthalmic use Initial U.S. Approval: 2001 INDICATIONS AND USAGEIZBA is a prostaglandin analog indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. (1) DOSAGE AND ADMINISTRATIONOne drop in the affected eye(s) once daily in the evening. (2) DOSAGE FORMS AND STRENGTHSOphthalmic solution containing 0.03 mg/mL travoprost. (3) CONTRAINDICATIONSNone. (4) WARNINGS AND PRECAUTIONSADVERSE REACTIONSMost common adverse reaction is ocular hyperemia as shown in a clinical trial for IZBA (12%). (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONSUse in pediatric patients below the age of 16 years is not recommended because of potential safety concerns related to increased pigmentation following long-term chronic use. (8.4) See 17 for PATIENT COUNSELING INFORMATION. Revised: 6/2021 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
IZBA® 0.003% is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
2 DOSAGE AND ADMINISTRATION
The recommended dosage is one drop in the affected eye(s) once daily in the evening. IZBA should not be administered more than once daily since it has been shown that more frequent administration of prostaglandin analogs may decrease the IOP-lowering effect.
Reduction of the IOP starts approximately 2 hours after the first administration with maximum effect reached after 12 hours.
IZBA may be used concomitantly with other topical ophthalmic drug products to lower IOP. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart.
5 WARNINGS AND PRECAUTIONS
5.1 Pigmentation
Travoprost ophthalmic solution has been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris, periorbital tissue (eyelid), and eyelashes. Pigmentation is expected to increase as long as travoprost is administered. The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. After discontinuation of travoprost, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes have been reported to be reversible in some patients. Patients who receive treatment should be informed of the possibility of increased pigmentation. The long term effects of increased pigmentation are not known.
Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. While treatment with IZBA can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
5.2 Eyelash Changes
IZBA may gradually change eyelashes and vellus hair in the treated eye. These changes include increased length, thickness, and number of lashes. Eyelash changes are usually reversible upon discontinuation of treatment.
5.3 Intraocular Inflammation
IZBA should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
5.4 Macular Edema
Macular edema, including cystoid macular edema, has been reported during treatment with travoprost ophthalmic solution. IZBA should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
5.5 Bacterial Keratitis
There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Different methodologies were used to collect adverse reactions during the development of travoprost. The most common adverse reaction observed in controlled clinical studies with travoprost 0.004% was ocular hyperemia. Ocular hyperemia was reported in 30% to 50% of patients by physician rating the severity of patient’s post treatment ocular hyperemia compared to standardized reference photographs and/or patients who discontinued therapy due to ocular hyperemia.
In a 3 month clinical trial involving 442 patients exposed to IZBA and 422 control patients exposed to travoprost ophthalmic solution, 0.004%, the most common adverse drug reaction was ocular hyperemia. This was reported in 12% of patients treated with IZBA based on clinical observations and/or patient complaints. One patient (0.2%) discontinued treatment with IZBA due to ocular hyperemia. Rates observed in the control patients were comparable.
Ocular adverse reactions reported in clinical studies with travoprost ophthalmic solutions, including IZBA at an incidence of 5% to 10% included decreased visual acuity, eye discomfort, foreign body sensation, pain, and pruritus. Ocular adverse reactions reported at an incidence of 1% to 4% included abnormal vision, blepharitis, blurred vision, cataract, conjunctivitis, corneal staining, dry eye, iris discoloration, keratitis, lid margin crusting, ocular inflammation, photophobia, subconjunctival hemorrhage, and tearing.
Nonocular adverse reactions reported at an incidence of 1% to 5% in these clinical studies were allergy, angina pectoris, anxiety, arthritis, back pain, bradycardia, bronchitis, chest pain, cold/flu syndrome, depression, dyspepsia, gastrointestinal disorder, headache, hypercholesterolemia, hypertension, hypotension, infection, pain, prostate disorder, sinusitis, urinary incontinence, and urinary tract infections.
In postmarketing use with prostaglandin analogs, periorbital and lid changes, including deepening of the eyelid sulcus have been observed.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of IZBA administration in pregnant women to inform a drug associated risk.
In animal reproduction studies, subcutaneous (SC) administration of travoprost to pregnant mice and rats throughout the period of organogenesis produced embryo-fetal lethality, spontaneous abortion, and premature delivery at potentially clinically relevant doses.
Advise pregnant women of a potential risk to a fetus. Because animal reproductive studies are not always predictive of human response, IZBA® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown; however, in the U.S. general population, the estimated background risk of major birth defects is 2%-4% and of miscarriage is 15%-20% of clinically recognized pregnancies.
Data
Animal Data
An embryo-fetal study was conducted in pregnant rats administered travoprost once daily by SC injection from gestation Day 6 to 17 to target the period of organogenesis. At 10 mcg/kg [81 times the maximum recommended human ocular dose (MRHOD), based on plasma Cmax)], travoprost was teratogenic in rats, evidenced by an increase in the incidence of skeletal malformations as well as external and visceral malformations, including fused sternebrae, domed head, and hydrocephaly. Travoprost caused post-implantation loss at 10 mcg/kg. The no observed adverse effect level (NOAEL) for post-implantation loss was 3 mcg/kg (24 times the MRHOD, based on estimated plasma Cmax). The maternal NOAEL was 10 mcg/kg.
An embryo-fetal study was conducted in pregnant mice administered travoprost once daily by SC injection from gestation Day 6 to 11, to target the period of organogenesis. At 1 mcg/kg (8.1 times the MRHOD, based on plasma Cmax), travoprost caused post-implantation loss and decreased fetal weight. The NOAEL for embryo-fetal toxicity was 0.3 mcg/kg (2.4 times the MRHOD, based on estimated plasma Cmax). The maternal NOAEL was 1 mcg/kg.
Pre/postnatal studies were conducted in rats administered travoprost once daily by SC injection from gestation Day 7 (early embryonic period) to postnatal Day 21 (end of lactation period). At doses of greater than or equal to 0.12 mcg/kg/day (0.97 times the MRHOD, based on estimated plasma Cmax), adverse pregnancy outcomes (embryo-fetal lethality, abortion, early delivery), low birth weight, and developmental delays were observed. The NOAEL for adverse pregnancy outcomes, low birth weight, and developmental delay was 0.1 mcg/kg (0.81 times the MRHOD, based on estimated plasma Cmax). The NOAEL for maternal toxicity was 0.72 mcg/kg (5.8 times the MRHOD, based on estimated plasma Cmax).
8.2 Lactation
Risk Summary
There are no data on the effects of travoprost on the breastfed child or milk production. It is not known if travoprost is present in human milk following ophthalmic administration. A study in lactating rats demonstrated that radiolabeled travoprost and/or its metabolites were transferred into milk following SC administration.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for use IZBA and any potential adverse effects on the breast-fed child from use of IZBA.
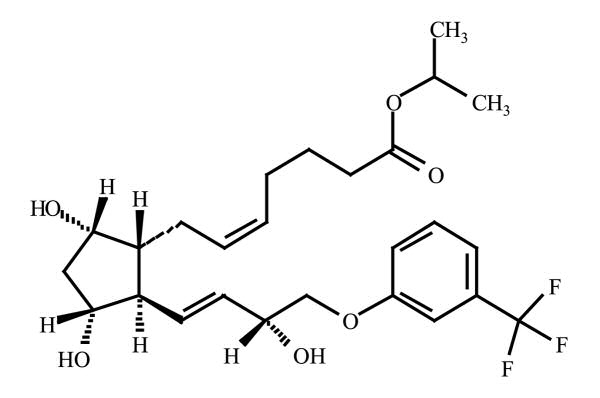
11 DESCRIPTION
Travoprost is a synthetic prostaglandin F2α analog. Its chemical name is [1R-[1α (Z),2ß (1E,3R*),3α ,5 α]]-7-[3,5-Dihydroxy-2-[3- hydroxy-4-[3-(trifluoromethyl) phenoxy]-1-butenyl]cyclopentyl]-5-heptenoic acid, 1-methylethylester. It has a molecular formula of C26H35F3O6 and a molecular weight of 500.55. The chemical structure of travoprost is:
Travoprost is a clear, colorless to slightly yellow oil that is very soluble in acetonitrile, methanol, octanol, and chloroform. It is practically insoluble in water.
IZBA is supplied as sterile, isotonic, buffered aqueous solution of travoprost with a pH of approximately 6.8 and an osmolality of approximately 290 mOsmol/kg.
IZBA contains Active: travoprost 0.03 mg/mL; Preservative: POLYQUAD* (polyquaternium-1) 0.01 mg/mL; Inactives: boric acid, mannitol, polyoxyethylene 40 hydrogenated castor oil, propylene glycol, purified water, sodium chloride, and sodium hydroxide and/or hydrochloric acid (to adjust pH).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Travoprost free acid, a prostaglandin analog is a selective FP prostanoid receptor agonist which is believed to reduce IOP by increasing uveoscleral outflow. The exact mechanism of action is unknown at this time.
12.3 Pharmacokinetics
Travoprost is absorbed through the cornea and is hydrolyzed to the active free acid. Data from four multiple dose pharmacokinetic studies using travoprost ophthalmic solution, 0.004% (totaling 107 subjects) have shown that plasma concentrations of the free acid are below 0.01 ng/mL (the quantitation limit of the assay) in two-thirds of the subjects. In those individuals with quantifiable plasma concentrations (N = 38), the mean plasma Cmax was 0.018 ± 0.007 ng/mL (ranged, 0.01 to 0.052 ng/mL) and was reached within 30 minutes. From these studies, travoprost is estimated to have a plasma half-life of 45 minutes. There was no difference in plasma concentrations between Days 1 and 7, indicating steady-state was reached early and that there was no significant accumulation.
Travoprost, an isopropyl ester prodrug, is hydrolyzed by esterases in the cornea to its biologically active free acid. Systemically, travoprost free acid is metabolized to inactive metabolites via beta-oxidation of the α(carboxylic acid) chain to give the 1,2-dinor and 1,2,3,4-tetranor analogs, via oxidation of the 15-hydroxyl moiety, as well as via reduction of the 13,14 double bond.
The elimination of travoprost free acid from plasma was rapid and levels were generally below the limit of quantification within one hour after dosing. The terminal elimination half-life of travoprost free acid was estimated from fourteen subjects and ranged from 17 minutes to 86 minutes with the mean half-life of 45 minutes. Less than 2% of the topical ocular dose of travoprost was excreted in the urine within 4 hours as the travoprost free acid.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Two-year carcinogenicity studies in mice and rats at SC doses of 10, 30, or 100 mcg/kg/day did not show any evidence of carcinogenic potential. However, at 100 mcg/kg/day, male rats were only treated for 82 weeks, and the maximum tolerated dose (MTD) was not reached in the mouse study. The high dose (100 mcg/kg) corresponds to exposure levels more than 400 times (mouse) and more than 700 times (rat) the human exposure at the MRHOD, based on estimated plasma Cmax for active travoprost free acid.
Mutagenesis
Travoprost was not mutagenic in the Ames test, mouse micronucleus test or rat chromosome aberration assay. A slight increase in the mutant frequency was observed in one of two mouse lymphoma assays in the presence of rat S-9 activation enzymes.
Impairment of Fertility
Travoprost did not affect mating or fertility indices in male or female rats at SC doses up to 3 mcg/kg/day (24 times the MRHOD, based on estimated plasma Cmax). At 10 mcg/kg/day (81 times the MRHOD, based on estimated plasma Cmax), the mean number of corpora lutea was reduced, and the post-implantation losses were increased.
14 CLINICAL STUDIES
A single clinical trial of 3 months duration was conducted to compare the IOP-lowering effect of IZBA® to TRAVATAN (travoprost ophthalmic solution) 0.004%, with both dosed once daily in the evening in adult patients with open angle glaucoma or ocular hypertension. Patient age ranged from 21 to 92 years, with a mean age of 65 years. A total of 864 patients (IZBA, 442 patients; TRAVATAN, 422 patients) were enrolled, with 840 (97%) completing through Month 3.
Analysis was based on the intent-to-treat (ITT) population defined as all patients who received study drug and completed at least one scheduled on-therapy study visit.
The least squares mean IOP (mmHg), the difference in mean IOP (IZBA minus TRAVATAN), and the 95% confidence interval (CI) for the treatment difference in mean IOP at visit and time point are presented in Table 1. The differences in the mean IOP at all visits and time points were within ± 1 mmHg, demonstrating equivalence of IZBA to TRAVATAN in lowering IOP.
Table 2 presents the mean IOP change from baseline at Week 2, Week 6, and at Month 3. IZBA demonstrated comparable IOP reductions at all on-therapy visits and time points; the mean IOP reduction from baseline in the IZBA group ranged from 7.1 to 8.2 mmHg and in the TRAVATAN group ranged from 7.1 to 8.4 mmHg. In both treatment groups, the greatest mean IOP reduction was observed at the 8 AM assessment time point.
| Visit/ Time Point | IZBA (travoprost 0.003%) | TRAVATAN (travoprost 0.004%) | Difference |
|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (95% CI)† | |
| Baseline | (N = 442) | (N = 418) | |
| 8 AM 10 AM 4 PM | 26.9 (0.12) 25.4 (0.13) 24.6 (0.14) | 27.1 (0.14) 25.6 (0.15) 24.8 (0.16) | -0.2 (-0.5, 0.2) -0.2 (-0.6, 0.2) -0.2 (-0.6, 0.2) |
| Week 2 | (N = 442) | (N = 416) | |
| 8 AM 10 AM 4 PM | 19.4 (0.16) 18.6 (0.16) 18.0 (0.16) | 19.5 (0.17) 18.6 (0.16) 18.3 (0.16) | -0.1 (-0.5, 0.3) -0.0 (-0.4, 0.4) -0.3 (-0.7, 0.1) |
| Week 6 | (N = 440**) | (N = 413) | |
| 8 AM 10 AM 4 PM | 19.3 (0.16) 18.5 (0.16) 18.0 (0.16) | 19.3 (0.17) 18.6 (0.17) 18.1 (0.17) | -0.0 (-0.4, 0.4) -0.1 (-0.5, 0.3) -0.2 (-0.6, 0.2) |
| Month 3 | (N = 432**) | (N = 408) | |
| 8 AM 10 AM 4 PM | 19.2 (0.17) 18.3 (0.17) 18.0 (0.16) | 19.3 (0.18) 18.6 (0.18) 18.0 (0.17) | -0.1 (-0.5, 0.3) -0.3 (-0.7, 0.1) 0.0 (-0.4, 0.4) |
Abbreviations: IOP, intraocular pressure; SE, Standard Error; CI = Confidence Interval.
†Estimates for Week 2, Week 6, and Month 3 are based on least squares means derived from a statistical model that accounts for correlated IOP measurements within patient where site and 8 AM baseline IOP stratum are in the model; estimates for Baseline visit at each time point are based on a two sample independent t-test procedure.
**One subject had missing data at 8 AM at Week 6; one subject had missing data at 4 PM at Month 3.
|
Visit |
N |
8 AM | IZBA 10 AM |
4 PM |
N |
8 AM | TRAVATAN 10 AM |
4 PM |
|
|---|---|---|---|---|---|---|---|---|---|
| Week 2 | Mean 95% CI | 442 | -0.8 (-8.3, -7.7) | -7.3 (-7.6, -7.0) | -7.1 (-7.4, -6.8) | 416 | -8.1 (-8.4, -7.8) | -7.5 (-7.8, -7.2) | -7.1 (-7.4, -6.8) |
| Week 6 | Mean 95% CI | 440† | -8.1 (-8.4, -7.9) | -7.4 (-7.6, -7.1) | -7.2 (-7.5, -6.9) | 413 | -8.3 (-8.7, -8.0) | -7.5 (-7.9, -7.2) | -7.2 (-7.5, -6.9) |
| Month 3 | Mean 95% CI | 432† | -8.2 (-8.6, -7.9) | -7.5 (-7.9, -7.2) | -7.1 (-7.4, -6.8) | 408 | -8.4 (-8.7, -8.1) | -7.6 (-7.9, -7.2) | -7.3 (-7.7, -7.0) |
†One subject had missing data at 8 AM at Week 6; one subject had missing data at 4 PM at Month 3.
16 HOW SUPPLIED/STORAGE AND HANDLING
IZBA® (travoprost ophthalmic solution) 0.003% is a sterile, isotonic, buffered, preserved, aqueous solution of travoprost (0.03 mg/mL) supplied in an oval package system.
IZBA is supplied as a 2.5 mL solution in a 4 mL bottle and a 5 mL solution in a 7.5 mL bottle. The dispenser bottles are made of polypropylene and fitted with a natural color polypropylene dropper tip and a turquoise polypropylene overcap. Tamper evidence is provided with a shrink band around the closure and neck area of the package.
2.5 mL fill NDC 0065-2800-25
5 mL fill NDC 0065- 2800-05
Storage: Store at 2°C to 25°C (36°F to 77°F). After opening, IZBA can be used until the expiration date on the bottle.
17 PATIENT COUNSELING INFORMATION
Potential for Pigmentation
Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Advise patients about the possibility of eyelid skin darkening, which may be reversible after discontinuation of IZBA [see Warnings and Precautions (5.1)].
Potential for Eyelash Changes
Advise patients about the possibility of eyelash and vellus hair changes in the treated eye during treatment with IZBA. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment [see Warnings and Precautions (5.2)].
Handling the Container
Instruct patients to avoid allowing the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to avoid contamination of the solution by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
When to Seek Physician Advice
Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection), have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician's advice concerning the continued use of IZBA.
Use With Contact Lenses
Contact lenses should be removed prior to instillation of IZBA and may be reinserted 15 minutes following its administration [see Warnings and Precautions (5.6)].
Use With Other Ophthalmic Drugs
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes between applications.
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
T2021-80
| IZBA
travoprost solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |
| Registrant - Alcon Laboratories, Inc. (008018525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alcon Research LLC | 007672236 | MANUFACTURE(0065-2800) | |