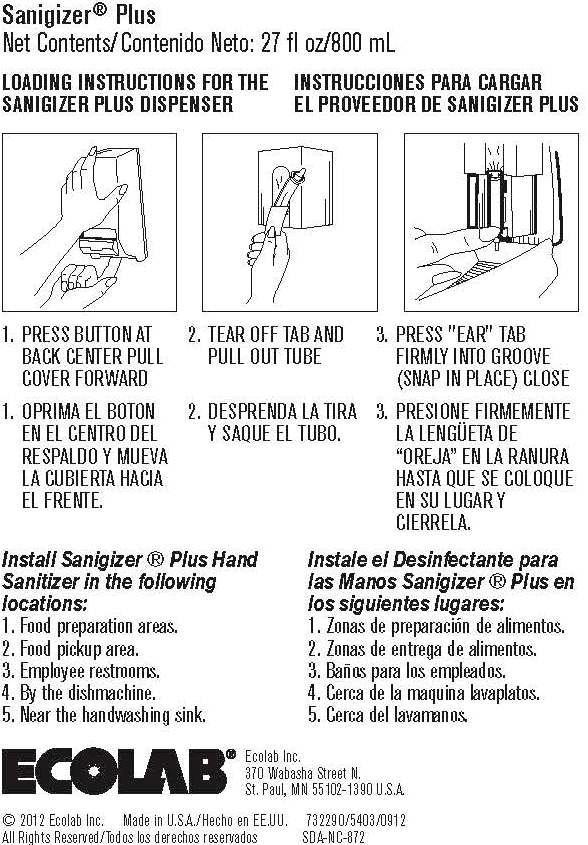
SANIGIZER PLUS- alcohol solution
Ecolab Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
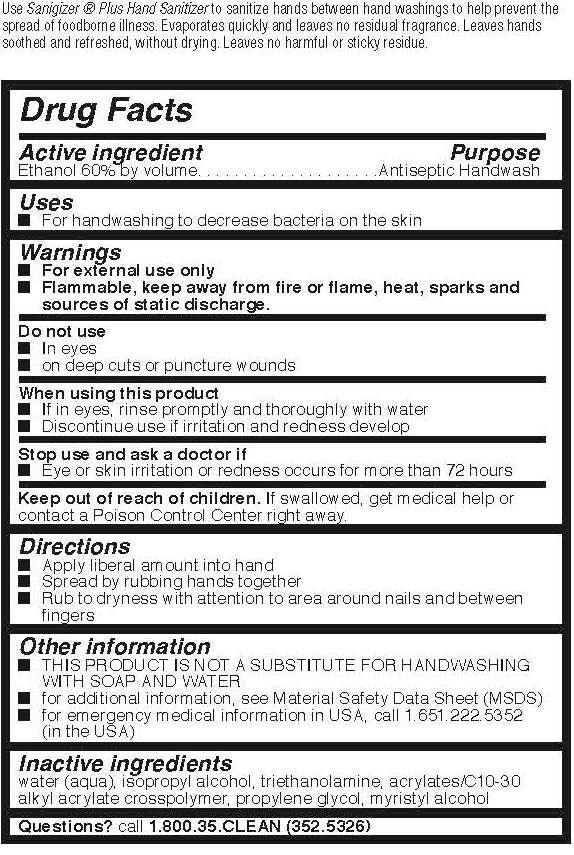
Drug Facts
Warnings
- For external use only
- FLAMMABLE. Keep away from fire or flame, heat, sparks and sources of static discharge.
Directions
- Apply Liberal amount into hand
- Spread by rubbing hands together
- Rub to dryness with attention to area around nails and between fingers
Other information
- THIS PRODUCT IS NOT A SUBSTITUTE FOR HANDWASHING WITH SOAP AND WATER.
- for additional information, see Material Safety Data Sheet (MSDS)
- for emergency medical information in USA, call 1.651.222.5352 (in the USA)
| SANIGIZER PLUS
alcohol solution |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Ecolab Inc. (006154611) |
Revised: 2/2018
Document Id: 97769635-b513-4279-9f62-345bc040bd2a
Set id: b49752dd-5e1a-4450-a115-67e1aa6d3bbf
Version: 4
Effective Time: 20180201
Ecolab Inc.