DERMAL WOUND CLEANSER- benzethonium chloride spray
Smith & Nephew, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Dermal Wound Cleanser
USES
- first aid to help reduce the risk of infection in minor cuts, scrapes and burns
- for washing small superficial wounds
- aids in the removal of foreign materials such as dirt and debris
WARNINGS
DIRECTIONS
- clean the affected area
- apply a small amount of this product on the area 1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
INACTIVE INGREDIENTS
benzyl alcohol, citric acid, disodium EDTA, glycerin, polyquaternium-10, polysorbate 20, sodium citrate, water
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - DERMAL WOUND CLEANSER BOTTLE, SPRAY (473mL)
Smith&Nephew
#449000
NDC 50484-490-00
Dermal
Wound
Cleanser
First Aid Antiseptic
- Helps reduce risk of infection in wounds and burns
- Gentle wound cleansing
- Safe and easy to use
- pH-buffered
- Non-irritating
16 fl. oz. (473mL)
Made for:
Smith & Nephew, Inc.
St. Petersburg, FL 33716
www.smith-nephew.com

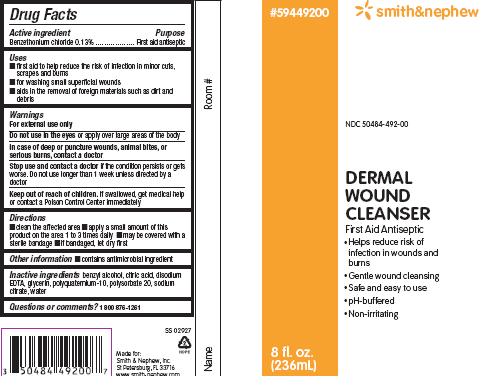
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - DERMAL WOUND CLEANSER BOTTLE, SPRAY (236mL)
Smith&Nephew
#59449200
NDC 50484-492-00
Dermal
Wound
Cleanser
First Aid Antiseptic
- Helps reduce risk of infection in wounds and burns
- Gentle wound cleansing
- Safe and easy to use
- pH-buffered
- Non-irritating
8 fl. oz. (236mL)
Made for:
Smith & Nephew, Inc.
St. Petersburg, FL 33716
www.smith-nephew.com
| DERMAL WOUND CLEANSER
benzethonium chloride spray |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| DERMAL WOUND CLEANSER
benzethonium chloride spray |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Smith & Nephew, Inc. (827731451) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Swiss-American CDMO, LLC | 080170933 | MANUFACTURE(50484-490, 50484-492) | |