Label: NITROGEN gas
-
Contains inactivated NDC Code(s)
NDC Code(s): 49972-002-01, 49972-002-02, 49972-002-03, 49972-002-04, view more49972-002-05, 49972-002-06, 49972-002-07, 49972-002-08, 49972-002-09, 49972-002-10, 49972-002-11, 49972-002-12, 49972-002-13, 49972-002-14, 49972-002-15, 49972-002-16, 49972-002-17, 49972-002-18, 49972-002-19, 49972-002-20, 49972-002-21, 49972-002-22, 49972-002-23, 49972-002-24, 49972-002-25, 49972-002-26, 49972-002-27, 49972-002-28, 49972-002-29, 49972-002-30, 49972-002-31, 49972-002-32, 49972-002-33, 49972-002-34, 49972-002-35 - Packager: Praxair Distribution Southeast LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved medical gas
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 16, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
UN166 NITROGEN, COMPRESSED NF
CONTENTS LOT NUMBER
CAUTION! HIGH-PRESSURE GAS. CAN CAUSE RAPID SUFFOCATION. MAY CAUSE DIZZINESS AND
(52°C). Use with equipment rated for cylinder pressure. Use a backflow prevention device in any piping. Close
valve after each use; keep closed even when empty. Always secure cylinder. Install cap, if provided, when not in use. Use per Praxair MSDS form P-4631 and safe practices booklets P-3499 and P-14-153; obtain from your local supplier. FIRST AID: IF INHALED, remove to fresh air. If not breathing, give artificial respiration. If breathing is
difficult, qualified personnel may give oxygen. Call a physician. WARNING! Administration of nitrogen may be
hazardous or contraindicated. For use only by or under the supervision of a licensed practitioner who is experienced in the use and administration of nitrogen and is familiar with the indications, effects, dosages, methods and frequency and duration of administration, and with the hazards, contraindications, and side effects and the precautions to be taken.
Rx ONLY CAS 7727-37-8
IN CASE OF EMERGENCY: CALL 1-800-645-4633
DISTRIBUTED BY PRAXAIR, INC., DANBURY
DO NOT REMOVE THIS LABEL PMG-4 (11/07)
Copyright @ 2002, 2005, 2007, Praxair Technology, inc.
Praxair, the flowing airstream design and Medipure are trademarks of Praxair Technology, Inc. DISTRIBUTED BY PRAXAIR, INC, DANBURY, CT 06810-5113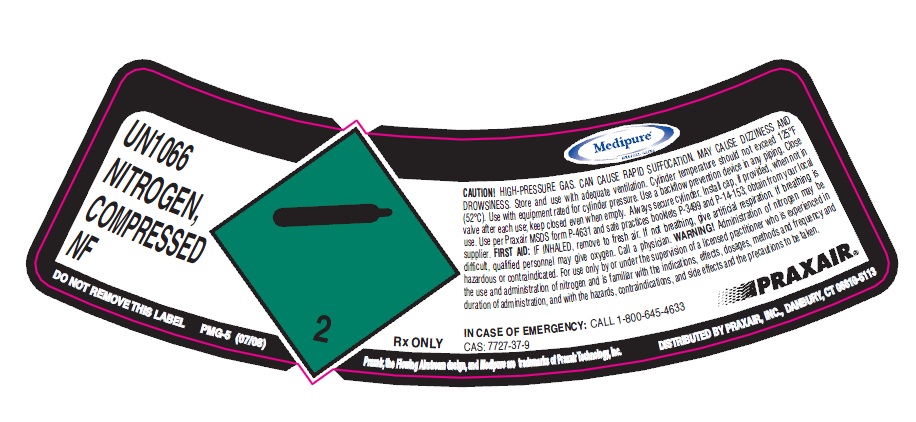
-
PRINCIPAL DISPLAY PANEL
UN1977 NITROGEN, REFRIGERATED LIQUID NF
ALWAYS KEEP CONTINER IN UPRIGHT POSITION. DO NOT CHANGE OR FORCE-FIT CONNECTIONS
LOT #
NET CONTENTS
WARNING! EXTREMELY COLD LIQUID AND GAS UNDER PRESSURE. CAN CAUSE RAPID SUFFOCATION. CAN CAUSE SEVERE FROSTBITE. MAY CAUSE DIZZINESS AND DROWSINESS.
Store and use with adequate ventilation. Do not get liquid in eyes, on skin, or on clothing. For liquid withdrawal, wear face shield and gloves. Do not drop. Use suitable hand truck for container movement. Use piping and equipment adequately designed to
withstand any pressures to be encountered. Use a backflow prevention device in any piping. Container temperature should not exceed 125°F (52°C). Close valve after each use; keep closed even when empty. Always secure container so it cannot be knocked over. Use per Praxair MSDS form P-4630 and safety booklets P-3499 and P-14-153 (obtain from your local supplier), and manufacturer's instructions for this container
RX ONLY
FIRST AID: IF INHALED, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, qualified personnel may give oxygen. Call a physician. IN CASE OF FROSTBITE, obtain medical treatment immediately.
WARNING: Administration of nitrogen may be hazardous or contraindicated. For use only by or under the supervision of a licensed practitioner who is experienced in the use and Administration of nitrogen and is familiar with the indications, effects, dosages, methods, and frequency and duration of administration, and with the hazards, contraindications, and side effects and the precautions to be taken.
DO NOT REMOVE THIS LABEL
Rx ONLY CAS 7727-37-9 PRODUCED BY AIR LIQUEFACTION
IN CASE OF EMERGENCY: CALL 1-800-645-4633
DISTRIBUTED BY PRAXAIR, INC., DANBURY
PMG-10 (07/08)
Copyright @ 1998, 2002, 2008. Praxair Technology, Inc. All rights reserved
Praxair, the flowing airstream design and Medipure are trademarks of Praxair Technology, Inc. DISTRIBUTED BY PRAXAIR, INC, DANBURY, CT 06810-5113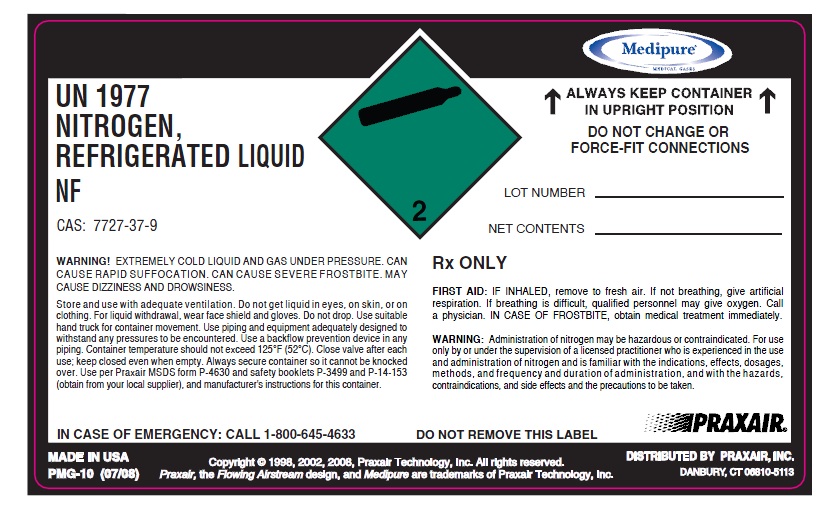
-
INGREDIENTS AND APPEARANCE
NITROGEN
nitrogen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49972-002 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nitrogen (UNII: N762921K75) (Nitrogen - UNII:N762921K75) Nitrogen 990 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49972-002-01 13819 L in 1 CYLINDER 2 NDC:49972-002-02 12176 L in 1 CYLINDER 3 NDC:49972-002-03 9656 L in 1 CYLINDER 4 NDC:49972-002-04 8608 L in 1 CYLINDER 5 NDC:49972-002-05 7221 L in 1 CYLINDER 6 NDC:49972-002-06 6454 L in 1 CYLINDER 7 NDC:49972-002-07 4021 L in 1 CYLINDER 8 NDC:49972-002-08 3228 L in 1 CYLINDER 9 NDC:49972-002-09 2152 L in 1 CYLINDER 10 NDC:49972-002-10 1104 L in 1 CYLINDER 11 NDC:49972-002-11 651 L in 1 CYLINDER 12 NDC:49972-002-12 538 L in 1 CYLINDER 13 NDC:49972-002-13 396 L in 1 CYLINDER 14 NDC:49972-002-14 198 L in 1 CYLINDER 15 NDC:49972-002-15 113 L in 1 CYLINDER 16 NDC:49972-002-16 7079 L in 1 CYLINDER 17 NDC:49972-002-17 4021 L in 1 CYLINDER 18 NDC:49972-002-18 878 L in 1 CYLINDER 19 NDC:49972-002-19 651 L in 1 CYLINDER 20 NDC:49972-002-20 566 L in 1 CYLINDER 21 NDC:49972-002-21 396 L in 1 CYLINDER 22 NDC:49972-002-22 142 L in 1 CYLINDER 23 NDC:49972-002-23 87773 L in 1 DEWAR 24 NDC:49972-002-24 92489 L in 1 DEWAR 25 NDC:49972-002-25 102281 L in 1 DEWAR 26 NDC:49972-002-26 107722 L in 1 DEWAR 27 NDC:49972-002-27 114976 L in 1 DEWAR 28 NDC:49972-002-28 132023 L in 1 DEWAR 29 NDC:49972-002-29 137463 L in 1 DEWAR 30 NDC:49972-002-30 247724 L in 1 DEWAR 31 NDC:49972-002-31 363063 L in 1 DEWAR 32 NDC:49972-002-32 91400 L in 1 DEWAR 33 NDC:49972-002-33 101556 L in 1 DEWAR 34 NDC:49972-002-34 124406 L in 1 DEWAR 35 NDC:49972-002-35 143267 L in 1 DEWAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved medical gas 10/01/2007 Labeler - Praxair Distribution Southeast LLC (008455552) Establishment Name Address ID/FEI Business Operations Praxair Distribution Southeast LLC 969017086 manufacture(49972-002) Establishment Name Address ID/FEI Business Operations Praxair Distribution Southeast LLC 192167567 manufacture(49972-002) Establishment Name Address ID/FEI Business Operations Praxair Distribution Southeast LLC 006734263 manufacture(49972-002)


