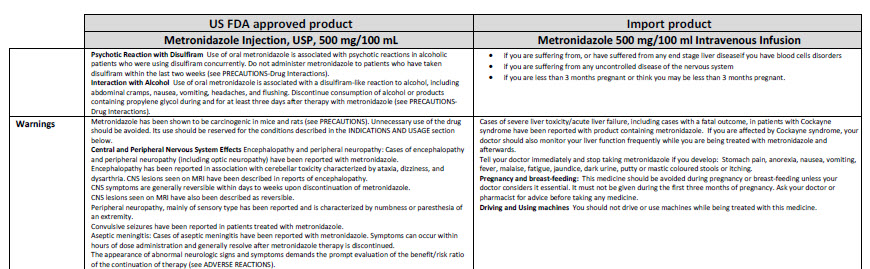
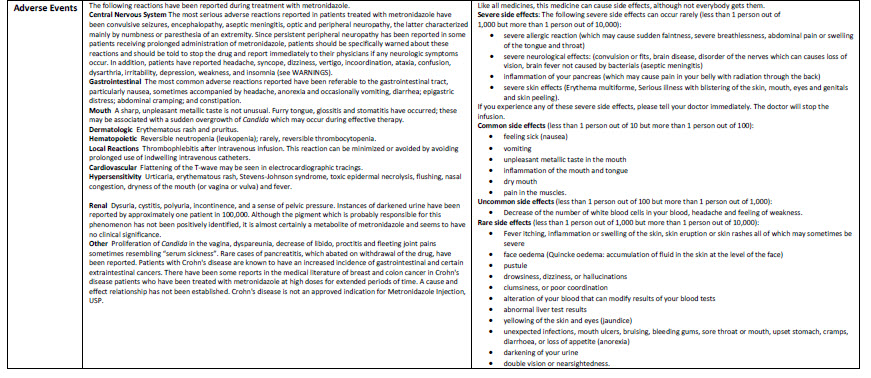
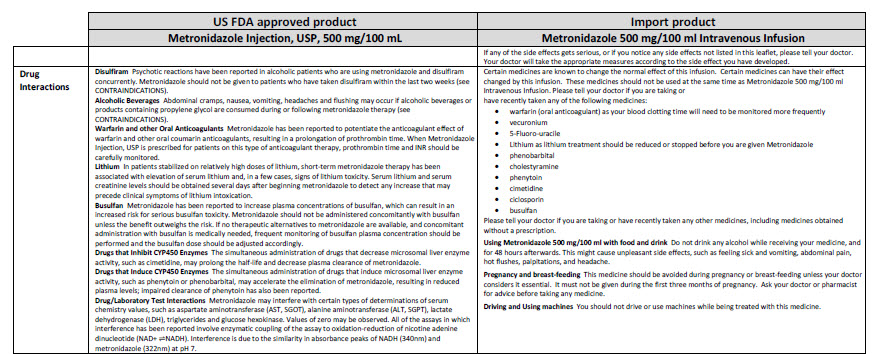
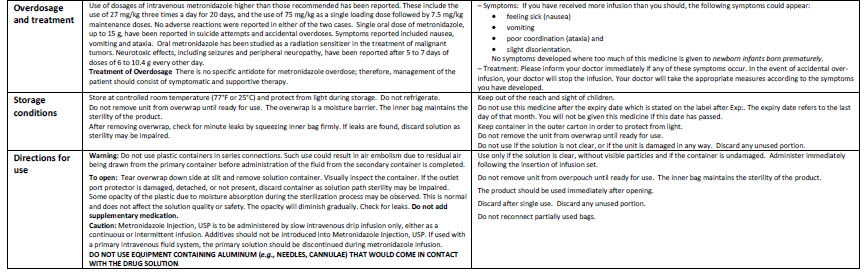
HEALTH CARE PROVIDER LETTER
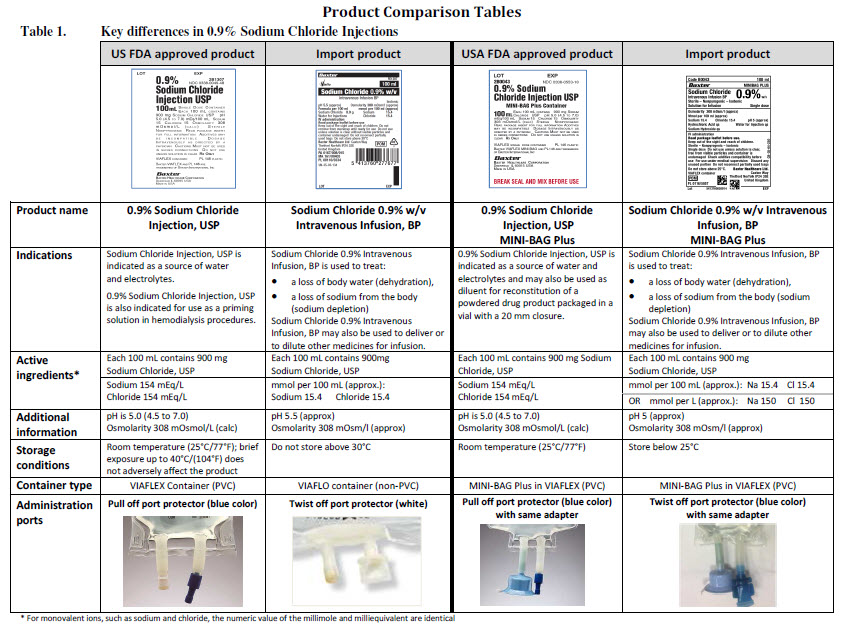
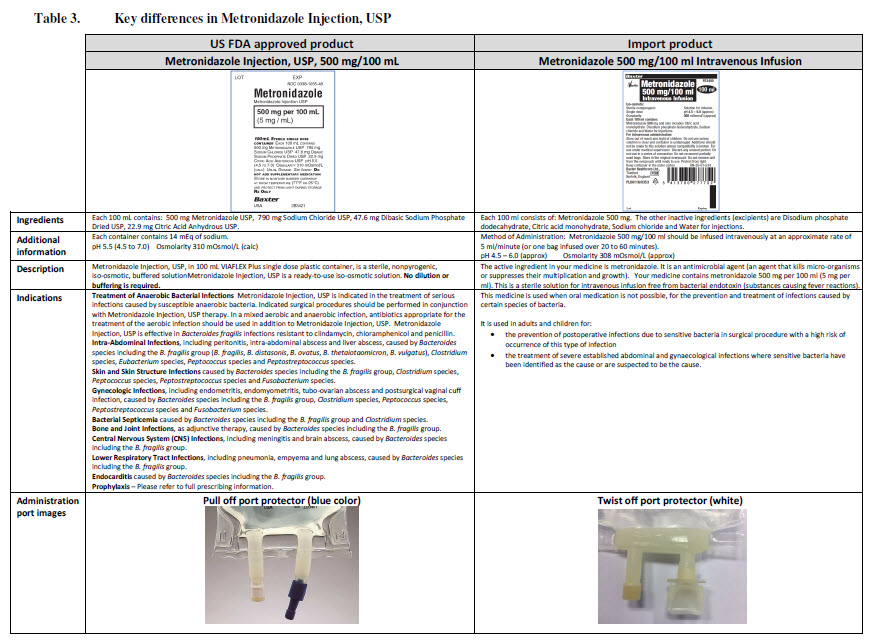
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Baxter Logo
FE0086G
Viaflo
50 ml
Glucose 5% w/v
Intravenous Infusion BP
pH 4.2 (approx.)
Isotonic
Osmolarity 278 m0sm/l (approx.)
Formula per 50 ml
Glucose (as monohydrate) 2.5 g Water for Injections
IV administration
Read package leaflet before use Keep out of the sight
and reach of children Do not remove from overwrap
until ready for use Do not administer simultaneously
with blood through the same infusion equipment Do not
use unless solution is clear without visible particles and
container undamaged Do not reconnect partially used
bags Do not store above 30ºC
Baxter Healthcare Ltd
Caxton Way Thetford Norfolk IP24 3SE POM 07
0
United Kingdom
PA 167/9/30
PL 00116/0335
MA 161/00205
UN-35-03-130
Bar Code
5 413760 277639
LOT
EXP
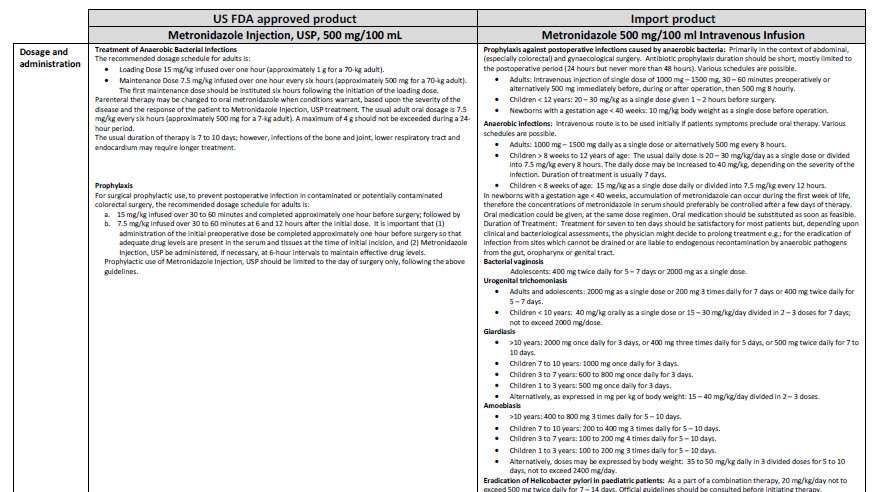
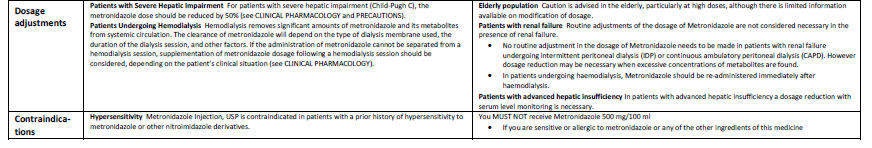
Baxter Logo
FE0087
Viaflo
100 ml
Glucose 5% w/v
Intravenous Infusion BP
pH 4.2 (approx.)
Isotonic
Osmolarity 278 m0sm/l (approx.)
Formula per 100 ml
Glucose (as monohydrate) 5.0 g Water for Injections
IV administration
Read package leaflet before use Keep out of the sight
and reach of children Do not remove from overwrap
until ready for use Do not administer simultaneously
with blood through the same infusion equipment Do not
use unless solution is clear without visible particles and
container undamaged Do not reconnect partially used
bags Do not store above 30ºC
Baxter Healthcare Ltd
Caxton Way Thetford Norfolk IP24 3SE POM 07
0
United Kingdom
PA 167/9/30
PL 00116/0335
MA 161/00205
UN-35-03-130
Bar Code
5 413760 277639
LOT
EXP