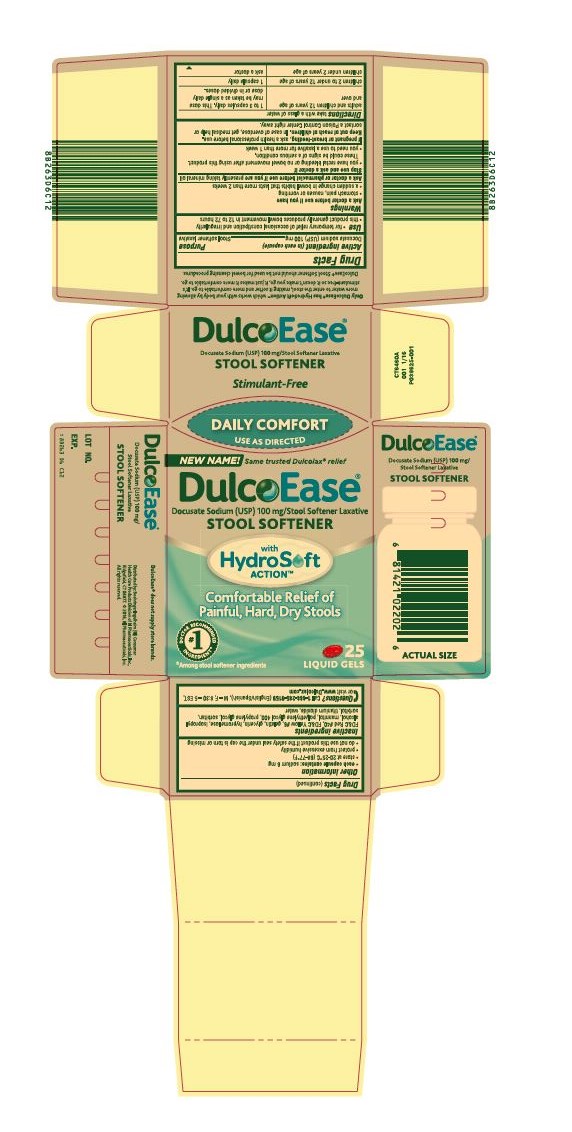
DULCOEASE- docusate sodium capsule, liquid filled
Boehringer Ingelheim Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DulcoEase®
Docusate
Sodium (USP) 100 mg/Stool Softener Laxative
- for temporary relief of occasional constipation and irregularity
- this product generally produces bowel movement in 12 to 72 hours
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
- you have rectal bleeding or no bowel movement after using this product. These could be signs of a serious condition.
- you need to use a laxative for more than 1 week
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions take with a glass of water
| adults and children 12 years of age and over | 1 to 3 capsules daily. This dose may be taken as a single daily dose or in divided doses. |
| children 2 to under 12 years of age | 1 capsule daily |
| children under 2 years of age | ask a doctor |
- each capsule contains: sodium 6 mg
- store at 20-25°C (68-77°F)
- protect from excessive humidity
- do not use this product if the safety seal under the cap is torn or missing
FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, hypromellose, isopropyl alcohol, mannitol, polyethylene glycol 400, propylene glycol, sorbitan, sorbitol, titanium dioxide, water
| DULCOEASE
docusate sodium capsule, liquid filled |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
Revised: 11/2021
Document Id: cec8c155-b504-4f70-8247-fb0b61dd5161
Set id: b1782087-d411-5cc9-3fe9-7e88c4b7eff8
Version: 4
Effective Time: 20211101
Boehringer Ingelheim Pharmaceuticals, Inc.