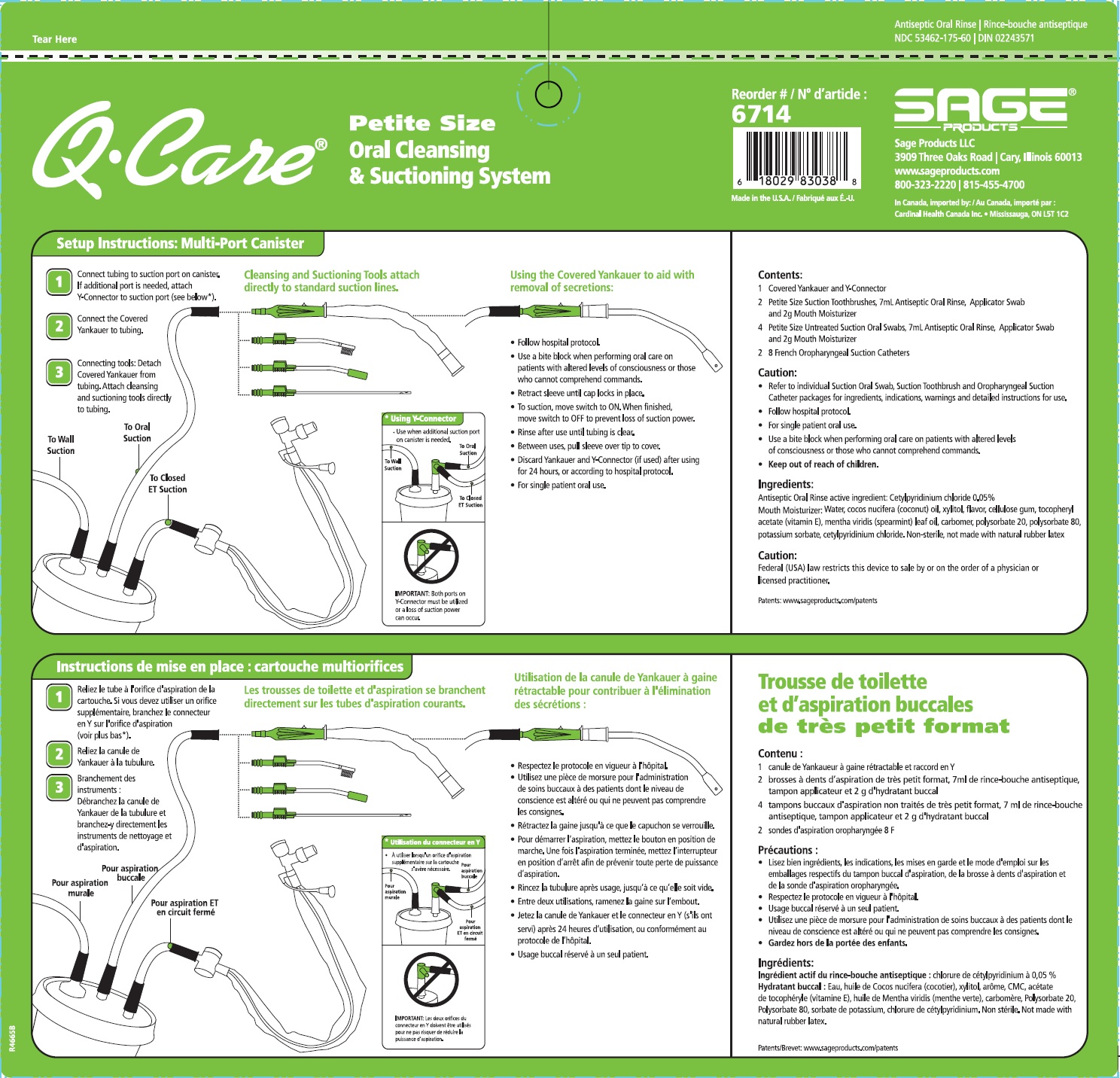
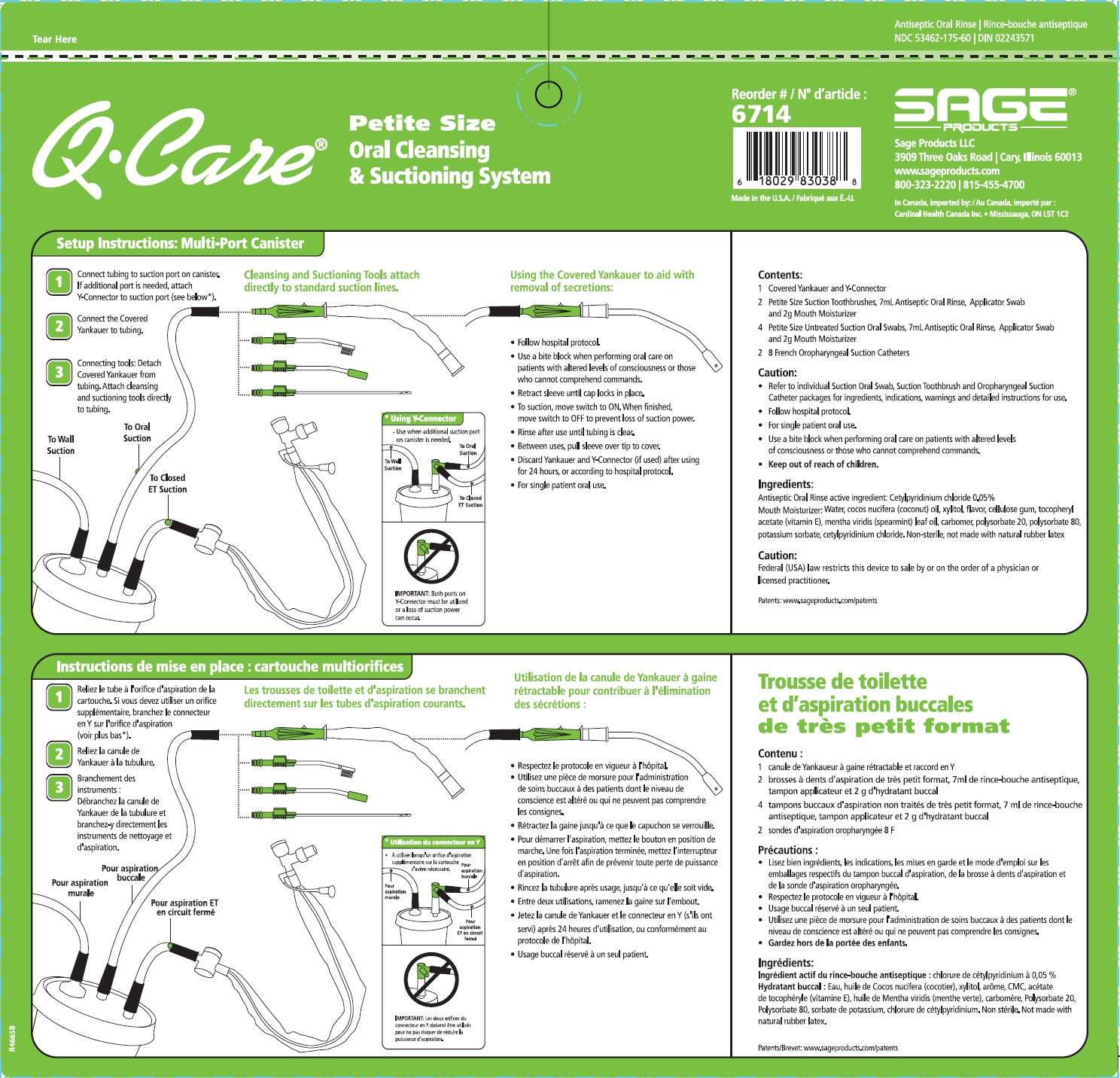
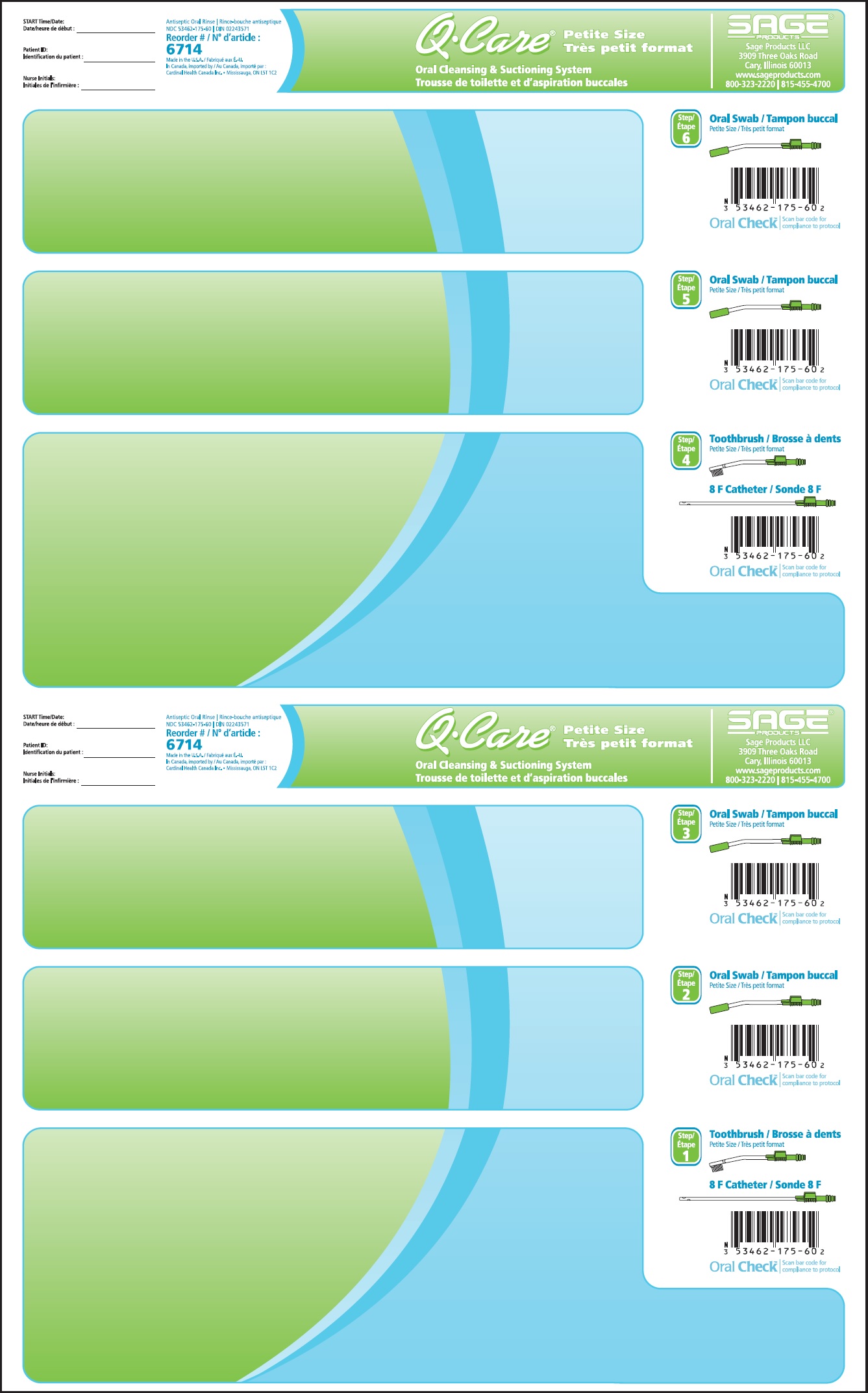
Label: QCARE PETITE SIZE ORAL CLEANSING AND SUCTIONING SYSTEM- cetylpyridinium chloride kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 53462-175-60, 53462-714-60 - Packager: Sage Products, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 17, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
-
Uses
Suction Swab with Antiseptic Oral Rinse
- Aids in the removal of secretions and debris and helps reduce the chance of infection in minor oral irritation.
Suction Toothbrush with Antiseptic Oral Rinse
- Aids in the removal of dental plaque, secretions, and debris; helps reduce the chance of infection in minor oral irritation.
Oropharyngeal Suction Catheter
- Aids in the removal of secretions from the oropharyngeal cavity only.
- INDICATIONS & USAGE
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
DIRECTIONS
Suction Swab with Antiseptic Oral Rinse
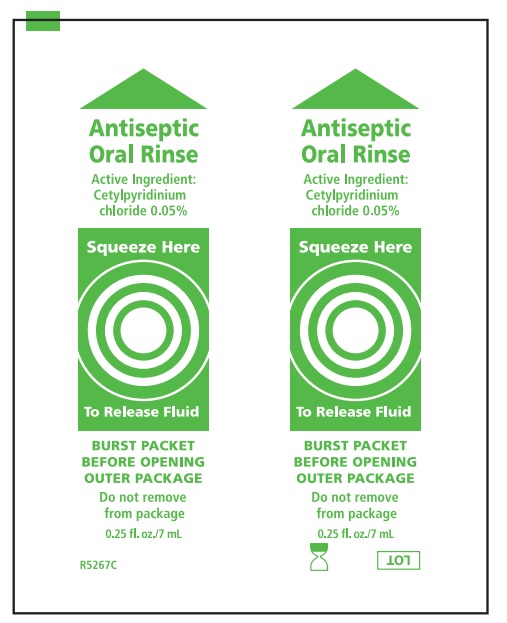
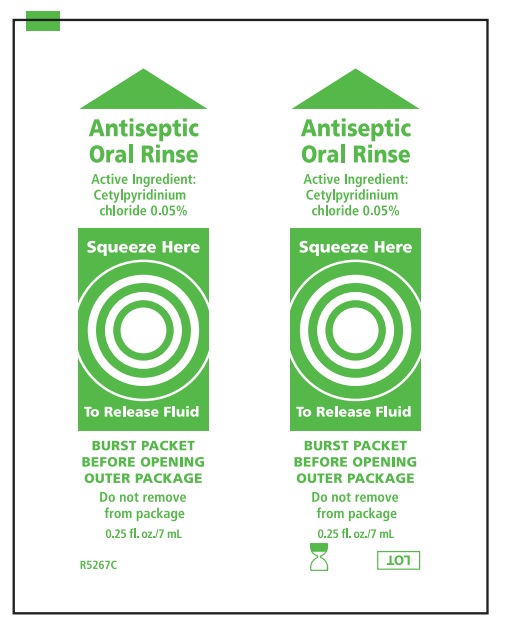
- Before opening, turn package over, burst solution packet with thumbs.
- Peel lid to open.
- Remove Mouth Moisturizer and Applicator Swab.
- Attach Suction Swab to suction line.
- Clean teeth and oral cavity for approximately one minute.
- To suction, place thumb over port.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Suction Swab. Reattach Covered Yankauer to suction line.
- Place Mouth Moisturizer on Applicator Swab.
- Apply as needed to lips and inside mouth.
- Use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age: supervise use.
- Children under 3 years of age: consult a dentist or doctor.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
Suction Toothbrush with Antiseptic Oral Rinse
- Before opening, turn package over, burst solution packet with thumbs.
- Peel lid to open.
- Remove Mouth Moisturizer and Applicator Swab.
- Attach Suction Toothbrush to suction line.
- Clean teeth and oral cavity for approximately one minute.
- To suction, place thumb over port.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Toothbrush. Reattach Covered Yankauer to suction line.
- Place Mouth Moisturizer on Applicator Swab.
- Apply as needed to lips and inside mouth.
- Use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age: supervise use.
- Children under 3 years of age: consult a dentist or doctor.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
Oropharyngeal Suction Catheter
- Peel lid to open.
- Attach Suction Catheter to suction line.
- To suction, place thumb over port.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Suction Catheter. Reattach Covered Yankauer to suction line.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
CAUTION:
- Federal (USA) law restricts this device to sale by or on the order of a physician or licensed practitioner.
- Inactive Ingredients
- Questions?
-
Mouth Moisturizer ingredients*
Water, cocos nucifera (coconut) oil, xylitol, flavor, cellulose gum, tocopheryl acetate (vitamin E), mentha veridis (spearmint) leaf oil, carbomer, polysorbate 20, polysorbate 80, potassium sorbate, cetylpyridinium chloride
* Manufactured for Sage Products LLC • Cary, IL
NOT MADE WITH NATURAL RUBBER LATEX • FOR SINGLE USE ONLY • MADE IN USA
- QCare Petite Size Oral Cleansing and Suctioning System
- Antiseptic Oral Rinse Solution
-
INGREDIENTS AND APPEARANCE
QCARE PETITE SIZE ORAL CLEANSING AND SUCTIONING SYSTEM
cetylpyridinium chloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53462-714 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53462-714-60 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 04/02/2013 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 PACKET 42 mL in 6 Part 2 6 PACKET 12 g in 6 Part 1 of 2 ANTISEPTIC ORAL RINSE
cetylpyridinium chloride mouthwashProduct Information Item Code (Source) NDC:53462-175 Route of Administration BUCCAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYSORBATE 20 (UNII: 7T1F30V5YH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6 in 1 KIT 1 NDC:53462-175-60 7 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 05/21/1998 Part 2 of 2 MOUTH MOISTURIZER
other oral hygiene products emulsionProduct Information Route of Administration BUCCAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR XYLITOL (UNII: VCQ006KQ1E) INGR CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR WATER (UNII: 059QF0KO0R) INGR CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR POLYSORBATE 80 (UNII: 6OZP39ZG8H) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6 in 1 KIT 1 2 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 04/02/2013 Labeler - Sage Products, LLC (054326178)