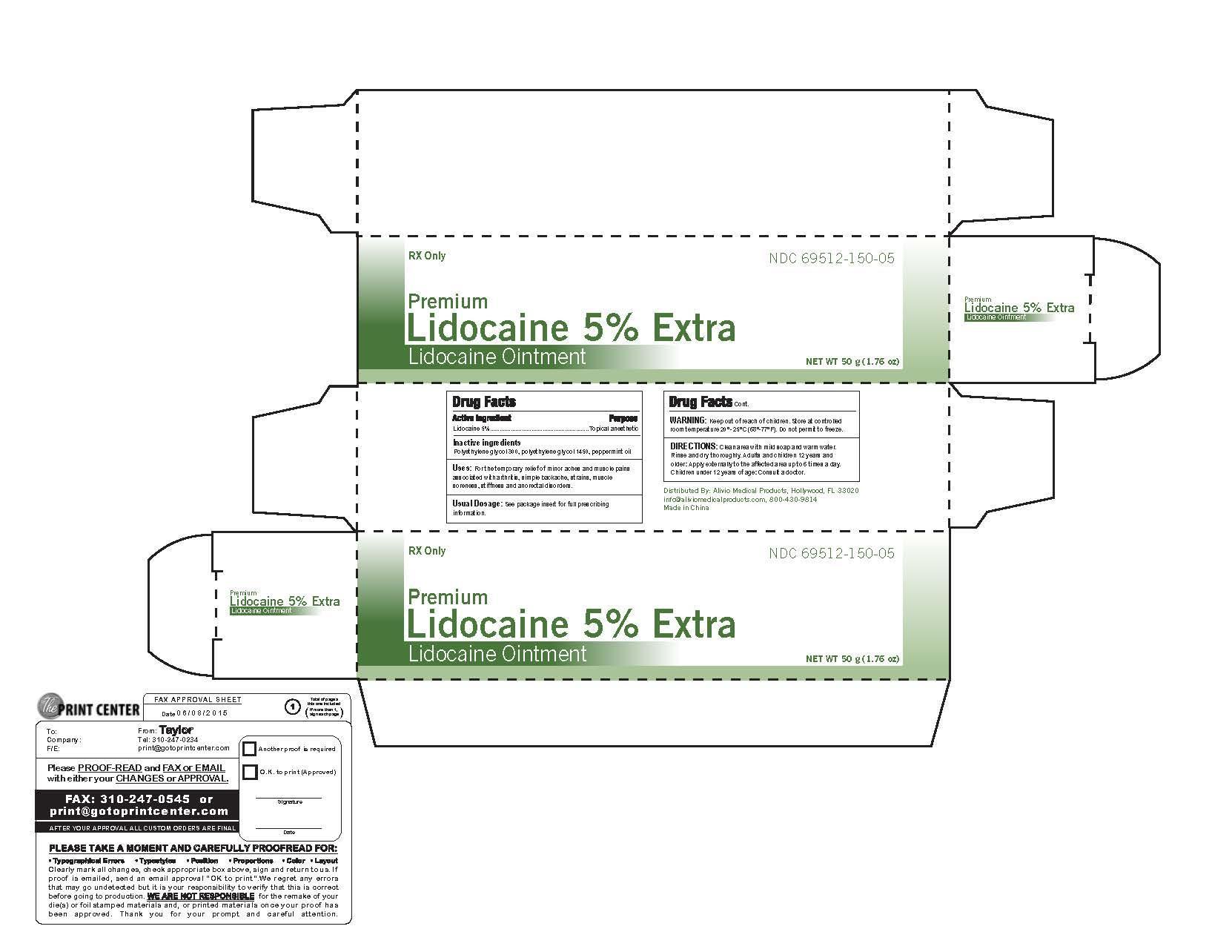
PREMIUM LIDOCAINE 5% EXTRA- lidocaine ointment
Zhejiang Bangli Medical Products Co., Ltd
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Drug Facts
Uses: For the temporary relief of minor aches and pains
associated with arthritis, simple backache, strain, muscle
soreness, stiffness and anorectal disorders
WARNING: Keep out of reach of children.
Store at controlled room temperature 20-25 C (68-77 F)
Do not permit to freeze
DIRECTIONS: Clean area with mild soap and water.
Rinse and dry thoroughly. Adults and children 12 years old
and older: Apply externally to the affected area up to 6 times a day.
Children under 12 years of age: Consult a doctor.
Lidocaine - lidocaine ointment
Alivio Medical Products, LLC
LIDOCAINE OINTMENT, 5%
FOR TOPICAL USE ONLY
Rx only
DESCRIPTION
Lidocaine Ointment 5% contains a local anesthetic agent and is administered topically. See INDICATIONS
AND USAGE for specific uses.
Lidocaine Ointment contains lidocaine, which is chemically designated as acetamide, 2-(diethylamino)-
N-(2,6-dimethylphenyl)-
Composition of Lidocaine Ointment 5%: acetamide, 2-(diethylamino)N-(2,6-dimethylphenyl)-(lidocaine)
5% in a water miscible ointment vehicle containing polyethylene glycols and peppermint oil.
CLINICAL PHARMACOLOGY
Mechanism of Action
conduction of impulses, thereby affecting local anesthetic action.
| PREMIUM LIDOCAINE 5% EXTRA
lidocaine ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Zhejiang Bangli Medical Products Co., Ltd (421295875) |