MEDI FIRST ANTACID- calcium carbonate tablet, chewable
MEDI FIRST PLUS ANTACID- calcium carbonate tablet, chewable
MEDIQUE ALCALAK- calcium carbonate tablet, chewable
Unifirst First Aid Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Medique® Alcalak
Uses
For the relief of the following symptoms associated with
- acid indigestion
- sour stomach
- heartburn
- upset stomach
Warnings
Directions
- do not use more than directed
Other information
- Phenylketonurics: contains phenylalanine 1.5 mg per tablet
- each tablet contains 168 mg of elemental calcium
- store at room temperature 59º-86º F (15º-30º C) in a dry place
- tamper-evident sealed packets
- do not use any opened or torn packets
Inactive ingredients
aspartame*, corn starch*, croscarmellose sodium*, D&C yellow #10*, FD&C blue #1*, magnesium stearate, mint flavor, silicon dioxide*, sorbitol*, starch*, sucrose*
*may contain
Principle Display Panel - 101R MF Antacid Label
250 Tablets
(125 x 2)
Medi-First®
Antacid Calcium Carbonate
Antiacido
Carbonato de Calcio
Pull to Open
Tire Para Abrir
Calcium Rich
Chewable Tablets
Tabletas Masticbles, Ricas en Calcio
Tamper Evident Unit Dose Packets
Empaquetado con Sellado
Evidente en Dosis Unitarias
Compare active ingredient to:
Compare el ingrediente activo con:
Tums®
Registered Trademark of Smithkline-Beecham
Marca Registrada de Smithkline-Beecham
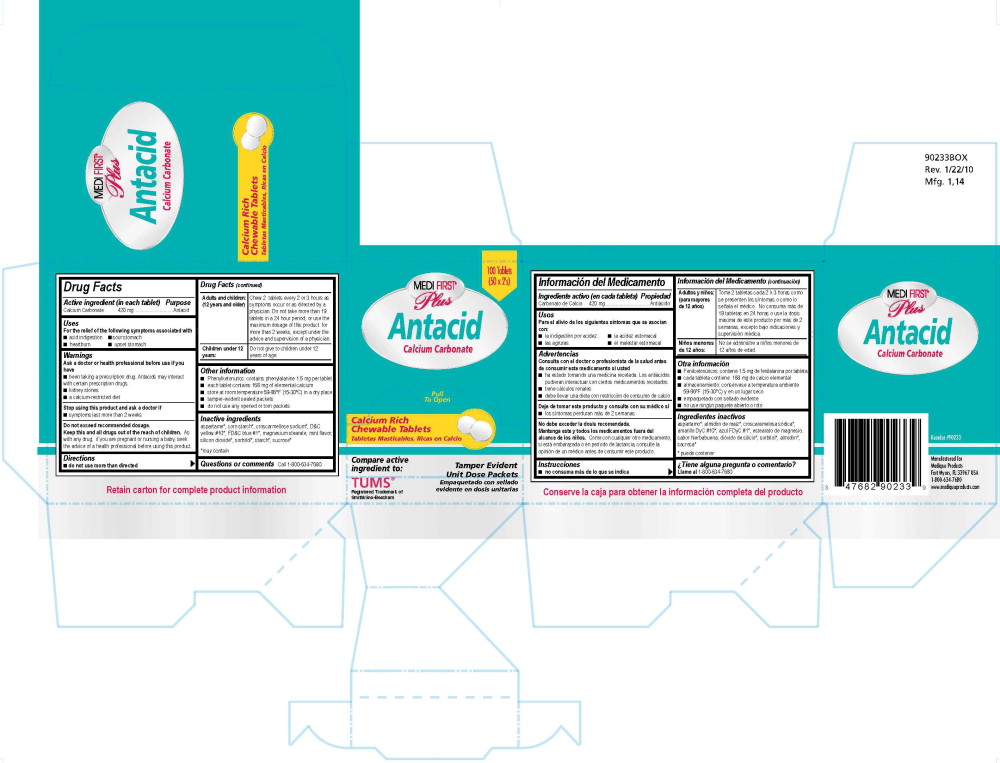
Principle Display Panel - 101R MFP Antacid Label
100 Tablets
(50 x 2's)
Medi-First® Plus
Antacid
Calcium Carbonate
Pull to Open
Tire Para Abrir
Calcium Rich
Chewable Tablets
Tabletas Masticbles, Ricas en Calcio
Tamper Evident Unit Dose Packets
Empaquetado con sellado
evidente en dosis unitarias
Compare active ingredient to:
Tums®
Registered Trademark of Smithkline-Beecham
Principle Display Panel - 101R Medique Alcalak Label
Medique®
Alcalak
Collect MediBucks
See inside flap for more details
Calcium-Rich Chewable Tablets
Tabletas Masticables, Ricasen Calcio
Heartburn, Indigestion • Calcium Carbonate 420 mg
las agruras, la indigstion • Carbonato de Calcio 420 mg
Pull to Open
Tire Para Abrir
Mint Flavor
Sabor de la menta
500
Tablets
(250 x 2)
Tamper Evident Unit Dose Packets
Empaquetado consellado evidente en
dosis unitarias

| MEDI FIRST ANTACID
calcium carbonate tablet, chewable |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| MEDI FIRST PLUS ANTACID
calcium carbonate tablet, chewable |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| MEDIQUE ALCALAK
calcium carbonate tablet, chewable |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Unifirst First Aid Corporation (832947092) |