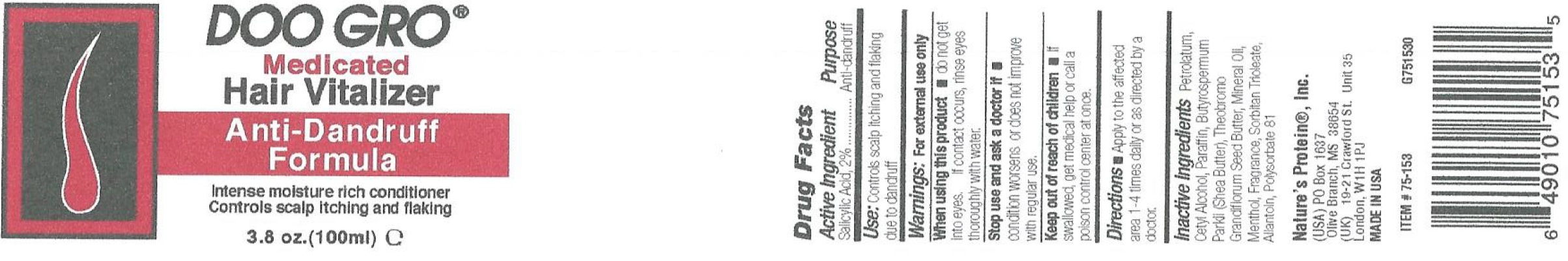
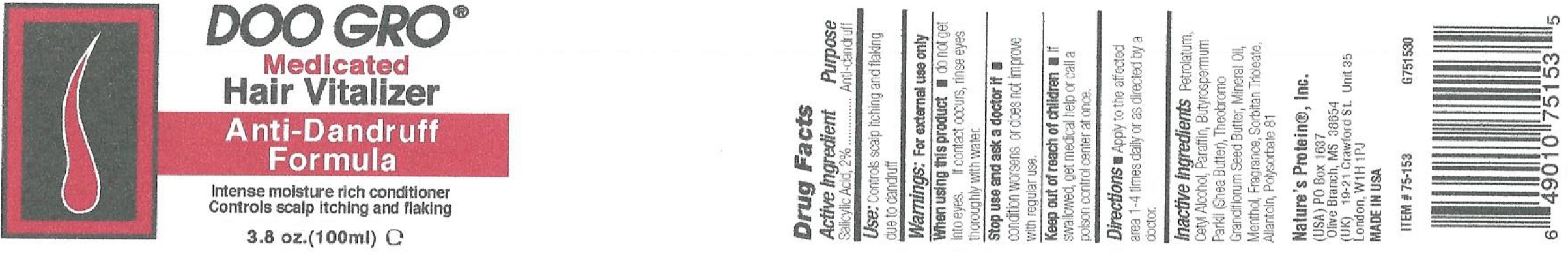
Label: DOO GRO ANTI-DANDRUFF VITALIZER- salicylic acid ointment
- NDC Code(s): 12022-006-00
- Packager: J. Strickland & Co. DBA Nature's Protein, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Use:
- Warnings:
- Directions
- Inactive Ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
DOO GRO ANTI-DANDRUFF VITALIZER
salicylic acid ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:12022-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) CETYL ALCOHOL (UNII: 936JST6JCN) PARAFFIN (UNII: I9O0E3H2ZE) SHEA BUTTER (UNII: K49155WL9Y) MINERAL OIL (UNII: T5L8T28FGP) MENTHOL (UNII: L7T10EIP3A) SORBITAN TRIOLEATE (UNII: QE6F49RPJ1) ALLANTOIN (UNII: 344S277G0Z) POLYSORBATE 81 (UNII: 2MSF640LWM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12022-006-00 100 mL in 1 JAR; Type 0: Not a Combination Product 01/08/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 01/08/2015 Labeler - J. Strickland & Co. DBA Nature's Protein, Inc. (007023112) Registrant - J. Strickland & Co. (007023112) Establishment Name Address ID/FEI Business Operations J. Strickland & Co. 007023112 manufacture(12022-006)