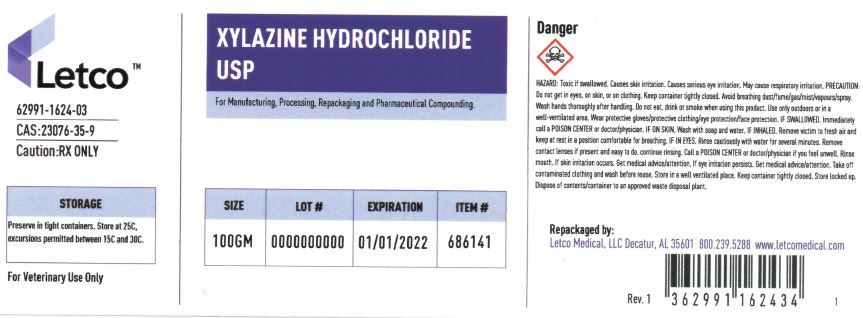
Label: XYLAZINE HYDROCHLORIDE powder
- NDC Code(s): 62991-1624-2, 62991-1624-3, 62991-1624-4
- Packager: LETCO MEDICAL, LLC
- Category: BULK INGREDIENT - ANIMAL DRUG
- DEA Schedule: None
- Marketing Status: Bulk Ingredient For Animal Drug Compounding
Drug Label Information
Updated April 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
XYLAZINE HYDROCHLORIDE
xylazine hydrochloride powderProduct Information Product Type Item Code (Source) NDC:62991-1624 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength XYLAZINE HYDROCHLORIDE (UNII: NGC3S0882S) (XYLAZINE - UNII:2KFG9TP5V8) XYLAZINE 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62991-1624-2 25 g in 1 JAR 2 NDC:62991-1624-3 100 g in 1 JAR 3 NDC:62991-1624-4 1000 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BULK INGREDIENT FOR ANIMAL DRUG COMPOUNDING 12/09/2011 Labeler - LETCO MEDICAL, LLC (079891426)