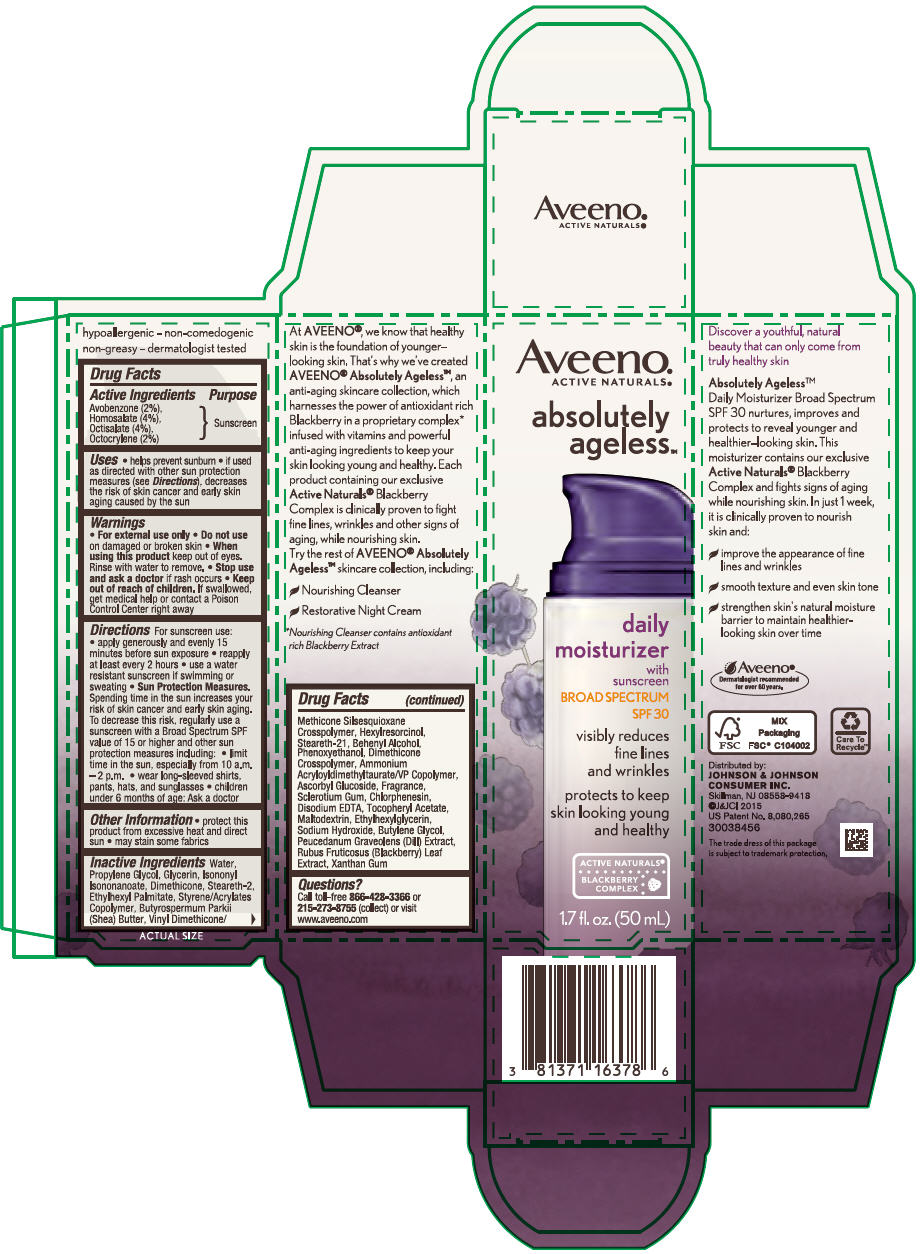
AVEENO ABSOLUTELY AGELESS DAILY MOISTURIZER BROAD SPECTRUM SPF30- avobenzone, homosalate, octisalate, and octocrylene lotion
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Aveeno® Absolutely Ageless™ Daily Moisturizer Broad Spectrum SPF 30
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
For sunscreen use:
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
Inactive ingredients
Water, Propylene Glycol, Glycerin, Isononyl Isononanoate, Dimethicone, Steareth-2, Ethylhexyl Palmitate, Styrene/Acrylates Copolymer, Butyrospermum Parkii (Shea) Butter, Vinyl Dimethicone/Methicone Silsesquioxane Crosspolymer, Hexylresorcinol, Steareth-21, Behenyl Alcohol, Phenoxyethanol, Dimethicone Crosspolymer, Ammonium Acryloyldimethyltaurate/VP Copolymer, Ascorbyl Glucoside, Fragrance, Sclerotium Gum, Chlorphenesin, Disodium EDTA, Tocopheryl Acetate, Maltodextrin, Ethylhexylglycerin, Sodium Hydroxide, Butylene Glycol, Peucedanum Graveolens (Dill) Extract, Rubus Fruticosus (Blackberry) Leaf Extract, Xanthan Gum
| AVEENO ABSOLUTELY AGELESS DAILY MOISTURIZER BROAD SPECTRUM SPF30
avobenzone, homosalate, octisalate, and octocrylene lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |