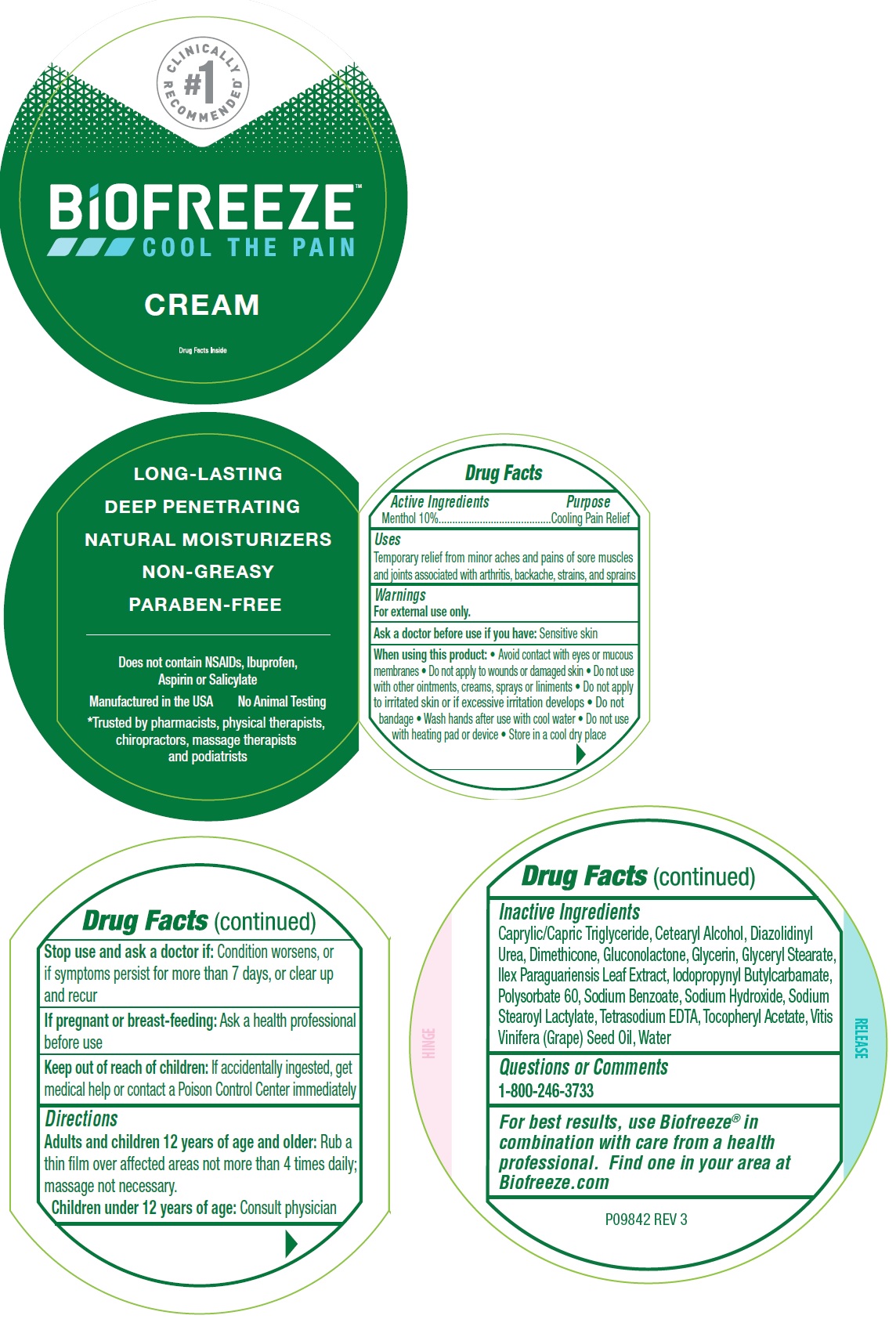
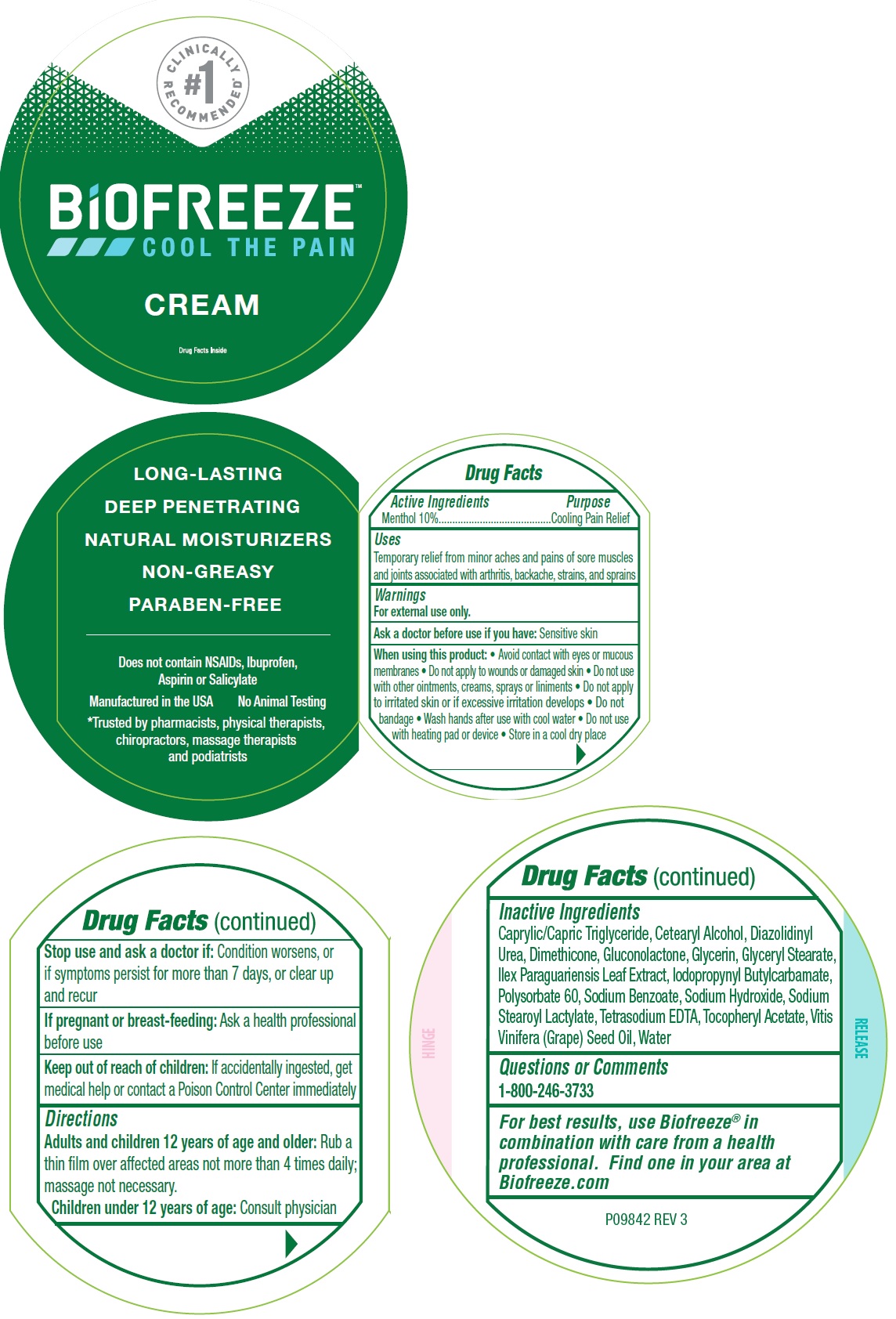
Label: BIOFREEZE PAIN RELIEF- menthol, unspecified form cream
- NDC Code(s): 59316-307-10
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 20, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingreidents
- Uses
-
Warnings
For external use only.
When using this product:
- Avoid contact with eyes or mucous membranes
- Do not apply to wounds or damaged skin
- Do not use with other ointments, creams, sprays or liniments
- Do not apply to irritated skin or if excessive irritation develops
- Do not bandage
- Wash hands after use with cool water
- Do not use with heating pad or device
- Store in a cool dry place
- Directions
-
Inactive Ingredients
Caprylic/Capric Triglyceride, Cetearyl Alcohol, Diazolidinyl Urea, Dimethicone, Gluconolactone, Glycerin, Glyceryl Stearate, Ilex Paraguariensis Leaf Extract, Iodopropynyl butylcarbamate, Polysorbate 60, Sodium Benzoate, Sodium Hydroxide, Sodium Stearoyl Lactylate, Tetrasodium EDTA, Tocopheryl Acetate, Vitis Vinifera (Grape) seed Oil, Water
- Questions or Comments
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
BIOFREEZE PAIN RELIEF
menthol, unspecified form creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59316-307 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength TRICAPRIN (UNII: O1PB8EU98M) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) DIMETHICONE (UNII: 92RU3N3Y1O) GLUCONOLACTONE (UNII: WQ29KQ9POT) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) POLYSORBATE 60 (UNII: CAL22UVI4M) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM STEAROYL LACTYLATE (UNII: IN99IT31LN) EDETATE SODIUM (UNII: MP1J8420LU) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GRAPE SEED OIL (UNII: 930MLC8XGG) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59316-307-10 1 in 1 CARTON 03/07/2018 12/31/2024 1 89 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/07/2018 12/31/2024 Labeler - RB Health (US) LLC (081049410)