VIRTPREX- ascorbic acid, cholecalciferol, .alpha.-tocopherol, d-, pyridoxine hydrochloride, folic acid, tribasic calcium phosphate, ferrous fumarate, doconexent and docusate sodi capsule, gelatin coated
Virtus Pharmaceuticals LLC
----------
VirtPrex
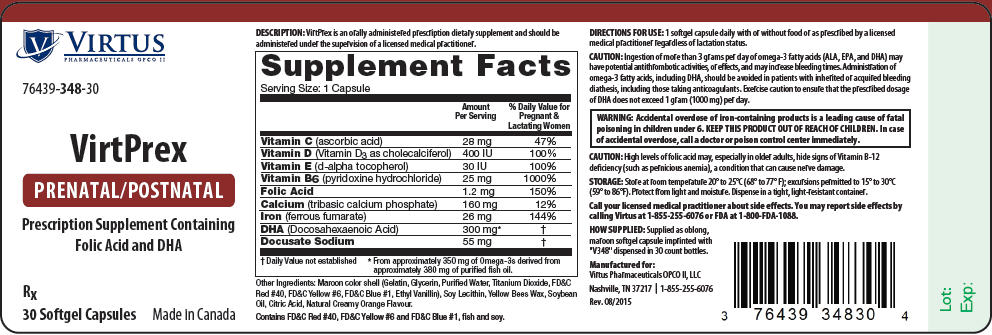
DESCRIPTION
VirtPrex is an orally administered prescription dietary supplement and should be administered under the supervision of a licensed medical practitioner.
| Supplement Facts Serving Size: 1 Capsule |
||
|---|---|---|
| Amount Per Serving | % Daily Value for Pregnant & Lactating Women | |
| Vitamin C (ascorbic acid) | 28 mg | 47% |
| Vitamin D (Vitamin D3 as cholecalciferol) | 400 IU | 100% |
| Vitamin E (d-alpha tocopherol) | 30 IU | 100% |
| Vitamin B6 (pyridoxine hydrochloride) | 25 mg | 1000% |
| Folic Acid | 1.2 mg | 150% |
| Calcium (tribasic calcium phosphate) | 160 mg | 12% |
| Iron (ferrous fumarate) | 26 mg | 144% |
| DHA (Docosahexaenoic Acid) | 300 mg* | † |
| Docusate Sodium | 55 mg | † |
Other Ingredients: Maroon color shell (Gelatin, Glycerin, Purified Water, Titanium Dioxide, FD&C Red #40, FD&C Yellow #6, FD&C Blue #1, Ethyl Vanillin), Soy Lecithin, Yellow Bees Wax, Soybean Oil, Citric Acid, Natural Creamy Orange Flavour.
Contains FD&C Red #40, FD&C Yellow #6 and FD&C Blue #1, fish and soy.
DIRECTIONS FOR USE
1 softgel capsule daily with or without food or as prescribed by a licensed medical practitioner regardless of lactation status.
CAUTION
Ingestion of more than 3 grams per day of omega-3 fatty acids (ALA, EPA, and DHA) may have potential antithrombotic activities, or effects, and may increase bleeding times. Administration of omega-3 fatty acids, including DHA, should be avoided in patients with inherited or acquired bleeding diathesis, including those taking anticoagulants. Exercise caution to ensure that the prescribed dosage of DHA does not exceed 1 gram (1000 mg) per day.
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
CAUTION
High levels of folic acid may, especially in older adults, hide signs of Vitamin B-12 deficiency (such as pernicious anemia), a condition that can cause nerve damage.
STORAGE
Store at room temperature 20° to 25°C (68° to 77° F); excursions permitted to 15° to 30°C (59° to 86°F). Protect from light and moisture. Dispense in a tight, light-resistant container.
Call your licensed medical practitioner about side effects. You may report side effects by calling Virtus at 1-855-255-6076 or FDA at 1-800-FDA-1088.
HOW SUPPLIED
Supplied as oblong, maroon softgel capsule imprinted with "V348" dispensed in 30 count bottles.
| VIRTPREX
ascorbic acid, cholecalciferol, .alpha.-tocopherol, d-, pyridoxine hydrochloride, folic acid, tribasic calcium phosphate, ferrous fumarate, doconexent and docusate sodi capsule, gelatin coated |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| flavor | ||
| scoring | 1 | |
| shape | ||
| size (solid drugs) | 19 mm | |
| imprint | ||
| Labeler - Virtus Pharmaceuticals LLC (969483143) |