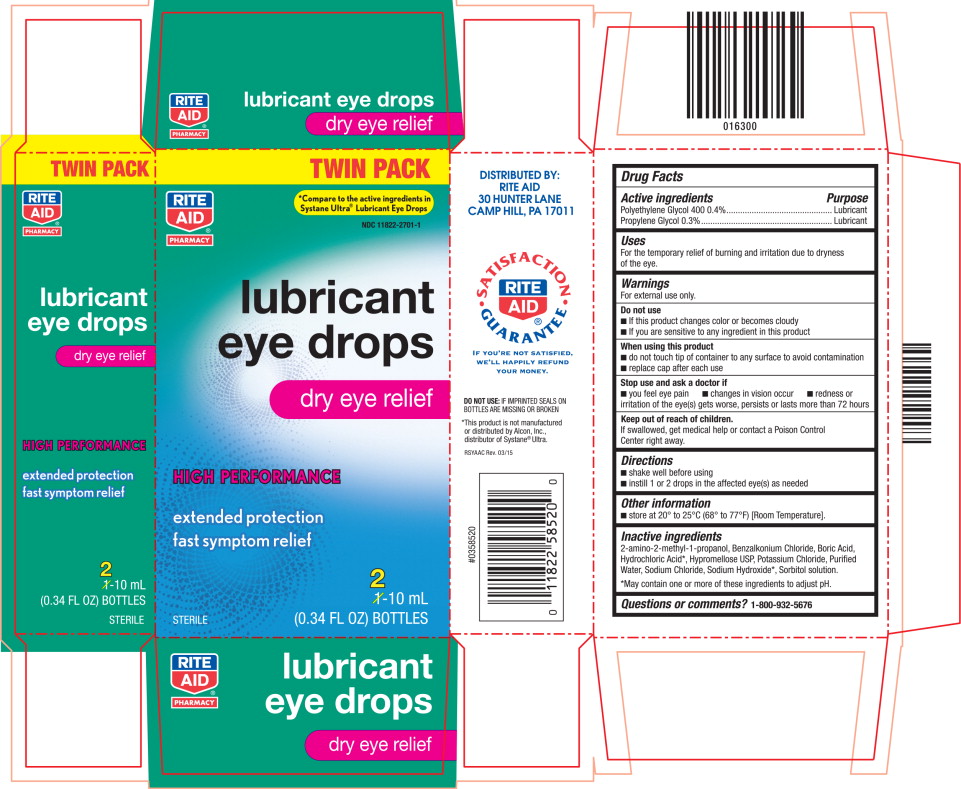
LUBRICANT EYE- polyethylene glycol 400 and propylene glycol solution/ drops
Rite Aid
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
For external use only.
Do not use
- If this product changes color or becomes cloudy
- If you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Inactive ingredients
2-amino-2-methyl-1-propanol, Benzalkonium Chloride, Boric Acid, Hydrochloric Acid*, Hypromellose USP, Potassium Chloride, Purified Water, Sodium Chloride, Sodium Hydroxide*, Sorbitol solution.
*May contain one or more of these ingredients to adjust pH.
| LUBRICANT EYE
polyethylene glycol 400 and propylene glycol solution/ drops |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Rite Aid (014578892) |
Revised: 10/2020
Document Id: 22098ca7-4013-4884-857b-021f45c80c8e
Set id: aacd7051-8f9c-4be4-98a6-62a314f7b71b
Version: 2
Effective Time: 20201021
Rite Aid