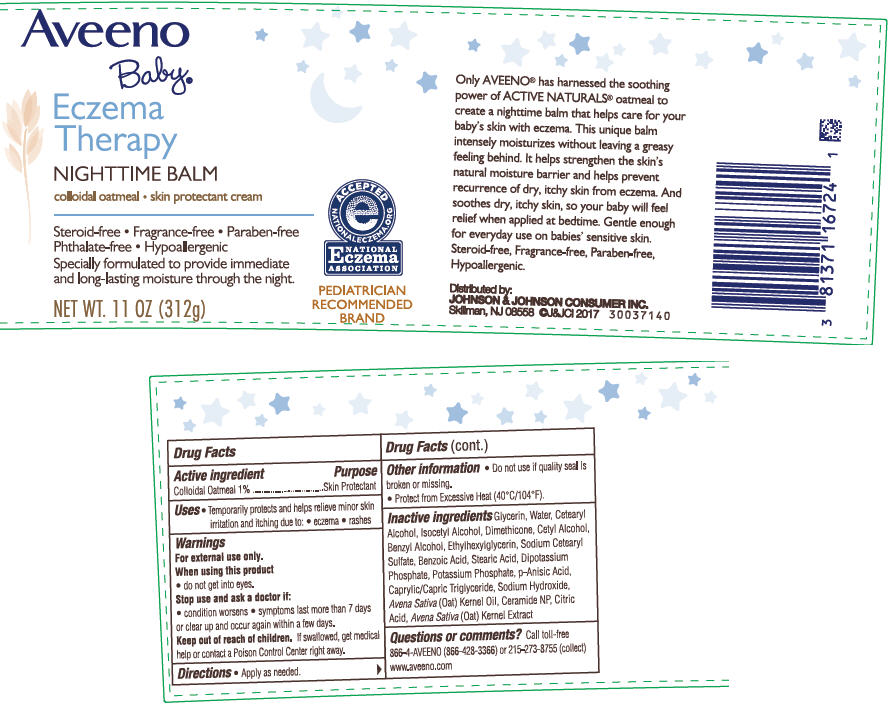
AVEENO BABY ECZEMA THERAPY NIGHTTIME BALM- oatmeal cream
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Aveeno Baby® Eczema Therapy NIGHTTIME BALM
Warnings
For external use only.
Other Information
- Do not use if quality seal is broken or missing.
- Protect from Excessive Heat (40°C/104°F).
Inactive Ingredients
Glycerin, Water ,Cetearyl Alcohol, Isocetyl Alcohol, Dimethicone, Cetyl Alcohol, Ethylhexylglycerin, Avena Sativa (Oat) Kernel Oil, Avena Sativa (Oat) Kernel Extract, Ceramide NP, Caprylic/Capric Triglyceride, Sodium Cetearyl Sulfate, Potassium Phosphate, Dipotassium Phosphate, Stearic Acid, Sodium Hydroxide, Citric Acid, Benzyl Alcohol, p –Anisic Acid, Benzoic Acid.
Questions or Comments?
Call toll-free 866-4AVEENO (866-428-3366) or 215-273-8755 (collect)
www.aveeno.com
PRINCIPAL DISPLAY PANEL - 312 g Jar Label
Aveeno
Baby®
Eczema
Therapy
NIGHTTIME BALM
colloidal oatmeal . skin protectant cream
Steroid-free . Fragrance-free . Paraben-free .
Phthalate-free . Hypoallergenic
Specially formulated to provide immediate
and long-lasting moisture through the night.
ACCEPTED
e
NATIONALECZEMA.ORG
NATIONAL
ECZEMA
ASSOCIATION
PEDIATRICIAN
RECOMMENDED
BRAND
NET WT. 11 OZ (312g)
| AVEENO BABY ECZEMA THERAPY NIGHTTIME BALM
oatmeal cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |