Label: FLAVOXATE HYDROCHLORIDE tablet, film coated
- NDC Code(s): 0574-0115-01
- Packager: Padagis US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Flavoxate HCl tablets contain flavoxate hydrochloride, a synthetic urinary tract spasmolytic.
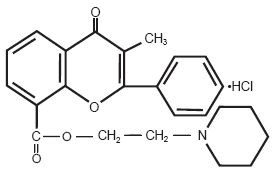
Chemically, flavoxate hydrochloride is 2-piperidinoethyl 3-methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylate hydrochloride. The empirical formula of flavoxate hydrochloride is C24H25NO4•HCl. The molecular weight is 427.94. The structural formula appears below:
Flavoxate HCl is supplied in tablets for oral administration. Each round, white, film-coated Flavoxate HCl tablet is debossed "PAD" and "0115" on one side and plain on the other side and contains flavoxate hydrochloride, 100 mg. Inactive ingredients consist of colloidal silicon dioxide, ethyl acrylate, hypromellose, lactose monohydrate, magnesium stearate, methyl methacrylate, microcrystalline cellulose, nonoxynol 100 and sodium starch glycolate. Film coating is composed of hypromellose 2910 6cP and polyethylene glycol.
-
CLINICAL PHARMACOLOGY
Flavoxate hydrochloride counteracts smooth muscle spasm of the urinary tract and exerts its effect directly on the muscle.
In a single study of 11 normal male subjects, the time to onset of action was 55 minutes. The peak effect was observed at 112 minutes. 57% of the flavoxate HCl was excreted in the urine within 24 hours.
-
INDICATIONS AND USAGE
Flavoxate HCl tablets are indicated for symptomatic relief of dysuria, urgency, nocturia, suprapubic pain, frequency and incontinence as may occur in cystitis, prostatitis, urethritis, urethrocystitis/urethrotrigonitis. Flavoxate HCl tablets are not indicated for definitive treatment, but are compatible with drugs used for the treatment of urinary tract infections.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Information for Patients:
Patients should be informed that if drowsiness and blurred vision occur, they should not operate a motor vehicle or machinery or participate in activities where alertness is required.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Mutagenicity studies and long-term studies in animals to determine the carcinogenic potential of flavoxate HCl have not been performed.
Pregnancy:
Teratogenic Effects-Pregnancy Category B.
Reproduction studies have been performed in rats and rabbits at doses up to 34 times the human dose and revealed no evidence of impaired fertility or harm to the fetus due to flavoxate HCl. There are, however, no well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
ADVERSE REACTIONS
The following adverse reactions have been observed, but there are not enough data to support an estimate of their frequency.
Gastrointestinal: Nausea, vomiting, dry mouth.
CNS: Vertigo, headache, mental confusion, especially in the elderly, drowsiness, nervousness.
Hematologic: Leukopenia (one case which was reversible upon discontinuation of the drug).
Cardiovascular: Tachycardia and palpitation.
Allergic: Urticaria and other dermatoses, eosinophilia and hyperpyrexia.
Ophthalmic: Increased ocular tension, blurred vision, disturbance in eye accommodation.
Renal: Dysuria.
- OVERDOSAGE
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Flavoxate HCl Tablets, 100 mg, are supplied as round, white, film-coated tablets debossed "PAD" and "0115" on one side and plain on the other side, in bottles of 100.
100 mg 100's:
NDC 0574-0115-01Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]
Manufactured For
Padagis®
Minneapolis, MN 55427
www.padagis.comRev 05-23
7H700 RC PH3
Code 917A00 - PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle
-
INGREDIENTS AND APPEARANCE
FLAVOXATE HYDROCHLORIDE
flavoxate hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0574-0115 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLAVOXATE HYDROCHLORIDE (UNII: 9C05J6089W) (FLAVOXATE - UNII:3E74Y80MEY) FLAVOXATE HYDROCHLORIDE 100 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ETHYL ACRYLATE (UNII: 71E6178C9T) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) METHYL METHACRYLATE (UNII: 196OC77688) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) NONOXYNOL-100 (UNII: A906T4D368) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color WHITE Score no score Shape ROUND Size 11mm Flavor Imprint Code PAD;0115 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0574-0115-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/22/2004 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076831 12/22/2004 Labeler - Padagis US LLC (967694121)