Label: 3M AVAGARD FOAMING INSTANT HAND ANTISEPTIC- alcohol liquid
- NDC Code(s): 17518-055-00, 17518-055-01, 17518-055-02
- Packager: Solventum US OpCo LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive Ingredients
- Questions?
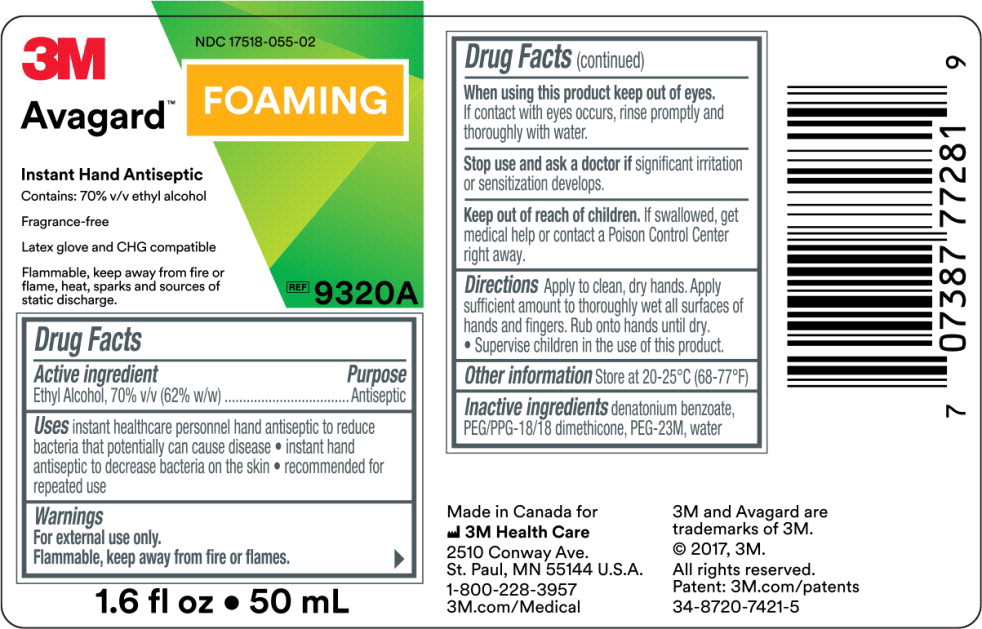
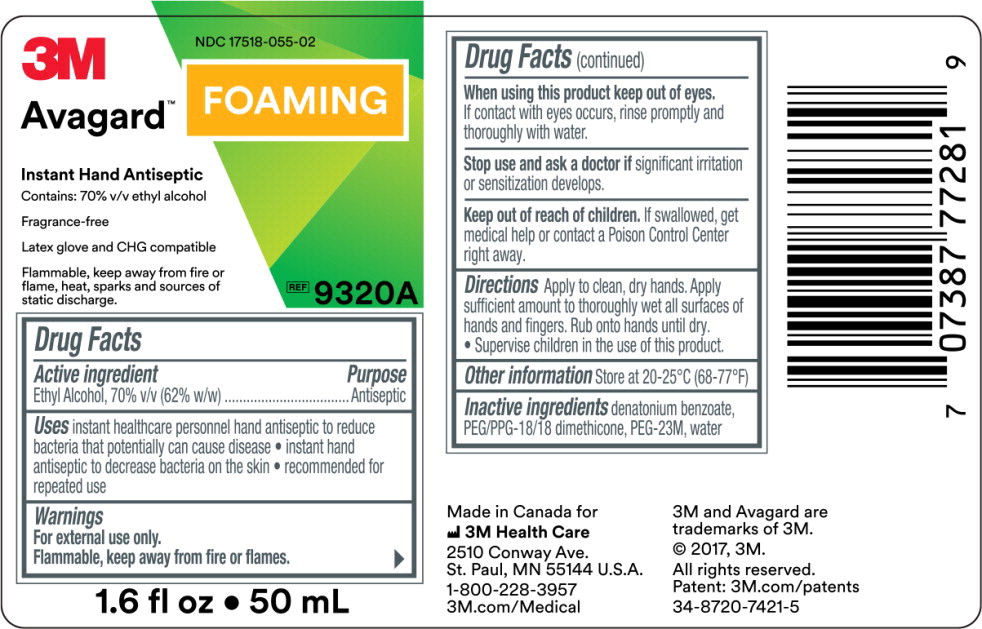
- Principal Display Panel – 50 mL Label
- Principal Display Panel – 500 mL Label
-
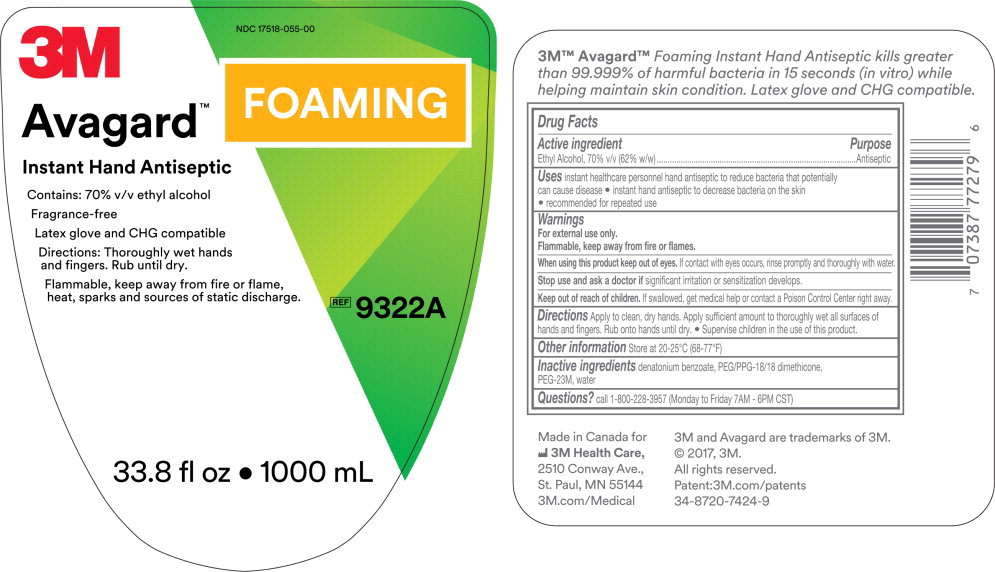
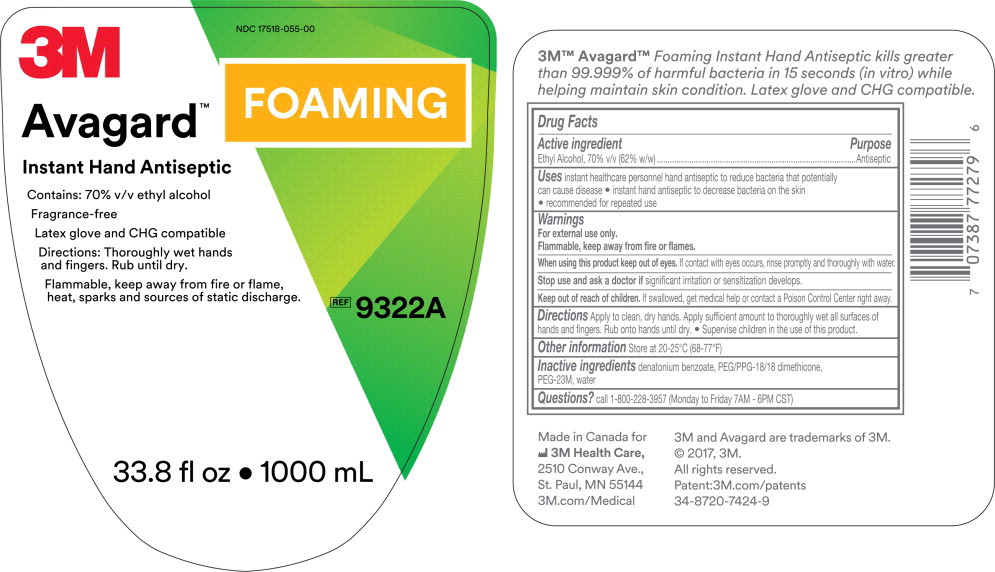
Principal Display Panel – 1000 mL Cartridge Label
3M
NDC 17518-055-00
Avagard™ Foaming
Instant Hand Antiseptic
Contains: 70% v/v ethyl alcohol
Fragrance-Free
Latex glove and CHG compatible
Directions: Thoroughly wet hands and fingers. Rub until dry.
Flammable, keep away from fire or flame, heat, sparks and sources of static discharge.
REF
9322A
33.8 fl oz • 1000 mL
-
INGREDIENTS AND APPEARANCE
3M AVAGARD FOAMING INSTANT HAND ANTISEPTIC
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17518-055 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Alcohol (UNII: 3K9958V90M) (Alcohol - UNII:3K9958V90M) Alcohol 548 mg in 1 mL Inactive Ingredients Ingredient Name Strength Denatonium Benzoate (UNII: 4YK5Z54AT2) PEG/PPG-18/18 Dimethicone (UNII: 9H0AO7T794) POLYETHYLENE OXIDE 1000000 (UNII: HZ58M6D839) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17518-055-02 25 in 1 CASE 05/27/2011 1 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:17518-055-01 12 in 1 CASE 05/27/2011 2 500 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 3 NDC:17518-055-00 5 in 1 CASE 05/27/2011 3 1000 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 05/27/2011 Labeler - Solventum US OpCo LLC (006173082)