L-METHYL PNV DHA- ascorbic acid, cholecalciferol, .alpha.-tocopherol, d-, thiamine mononitrate, riboflavin, niacinamide, pyridoxal 5-phosphate, levomefolate calcium, methylcobalamin, calcium carbonate, ferrous fumarate, potassium iodide, magnesium oxide, selenium and crypthecodinium cohnii dha oil capsule capsule, gelatin coated
Virtus Pharmaceuticals LLC
----------
L-Methyl PNV DHA
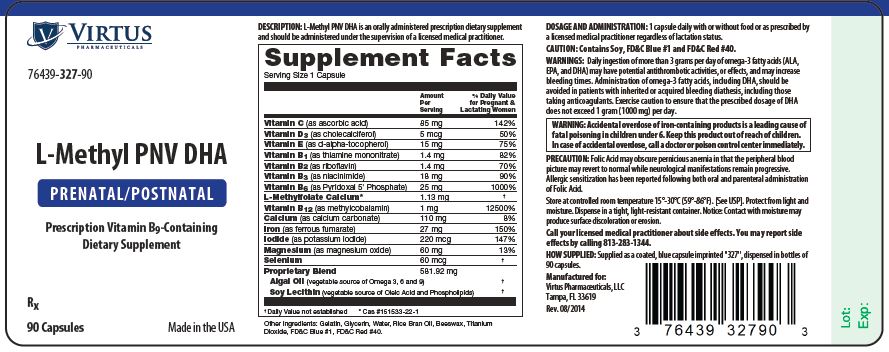
STATEMENT OF IDENTITY: L-Methyl PNV DHA is an orally administered prescription dietary supplement and should be administered under the supervision of a licensed medical practitioner.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
DOSAGE AND ADMINISTRATION: 1 capsule daily with or without food or as prescribed by a licensed medical practitioner regardless of lactation status.
|
|
| Vitamin C (as ascorbic acid) | 85 mg |
| Vitamin D3 (as cholecalciferol) | 5 mcg |
| Vitamin E (as d-alpha-tocopherol) | 15 mg |
| Vitamin B1 (as thiamine mononitrate) | 1.4 mg |
| Vitamin B2 (as riboflavin) | 1.4 mg |
| Vitamin B3 (as niacinimide) | 18 mg |
| Vitamin B6 (as Pyridoxal 5' Phosphate) | 25 mg |
| L-Methylfolate Calcium* | 1.13 mg |
| Vitamin B12 (as methylcobalamin) | 1 mg |
| Calcium (as calcium carbonate) | 110 mg |
| Iron (as ferrous fumarate) | 27 mg |
| Iodide (as potassium iodide) | 220 mcg |
| Magnesium (as magnesium oxide) | 60 mg |
| Selenium | 60 mcg |
| Crypthecodinium cohnii dha oil | 581.92 |
Other Ingredients: Soybean Lecithin, Gelatin, Glycerin, Water, Rice Bran Oil, Beeswax, Titanium Dioxide, FD&C Blue #1, FD&C Red #40.
How Supplied: Supplied as a coated, blue capsule imprinted "327", dispensed in bottles of 90 capsules.
PRECAUTIONS/WARNING: Folic Acid may obscure pernicious anemia in that the peripheral blood picture may revert to normal while neurological manifestations remain progressive. Allergic sensitization has been reported following both oral and parenteral administration of Folic Acid.
| L-METHYL PNV DHA
ascorbic acid, cholecalciferol, .alpha.-tocopherol, d-, thiamine mononitrate, riboflavin, niacinamide, pyridoxal 5-phosphate, levomefolate calcium, methylcobalamin, calcium carbonate, ferrous fumarate, potassium iodide, magnesium oxide, selenium and crypthecodinium cohnii dha oil capsule capsule, gelatin coated |
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| scoring | 1 | |
| shape | ||
| size (solid drugs) | 19 mm | |
| imprint | ||
| Labeler - Virtus Pharmaceuticals LLC (969483143) |