BACLEZENE- neomycin sulfate cream
Curazene, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
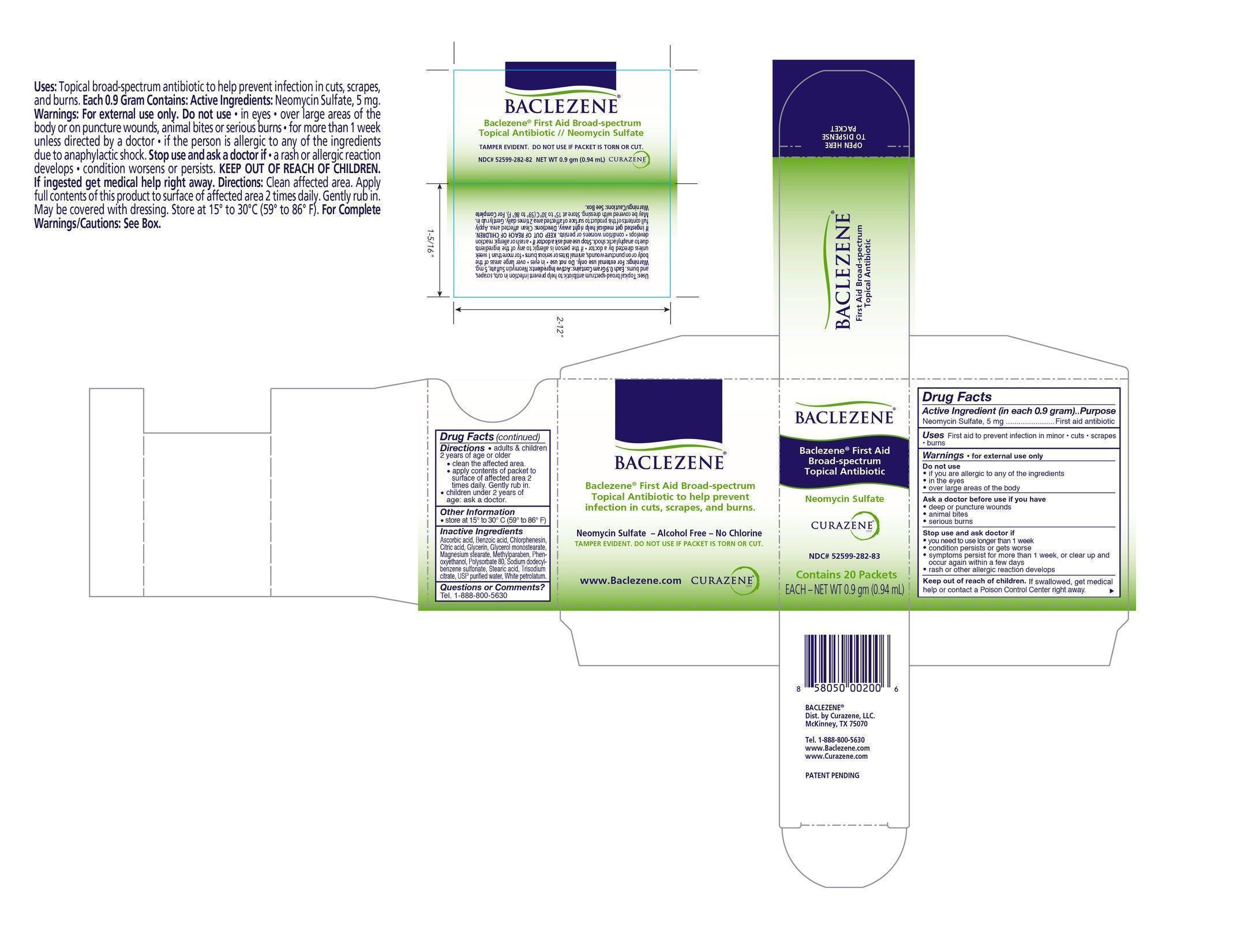
Drug Facts
Warnings: For external use only. Do not use: if you are allergic to any of the ingredients, in the eyes, over large areas of the body. Ask a doctor before use if you have: large puncture wounds, animal bites, serious burns.
Stop use and ask a doctor if: you need to use longer than one week, condition persists or gets worse, symptoms persist for more than one week or clear up and occur again within a few days, rash or other allergic reaction develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Adults and children over 2 years of age or older: Clean the affected area. Apply contents of packet to surface of affected area 2 times daily. Gently rub in. Children under 2 years of age: Ask a doctor.
| BACLEZENE
neomycin sulfate cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Curazene, LLC (005078008) |
| Registrant - Curazene, LLC (005078008) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ultra Seal Corporation | 085752004 | pack(52599-282) | |