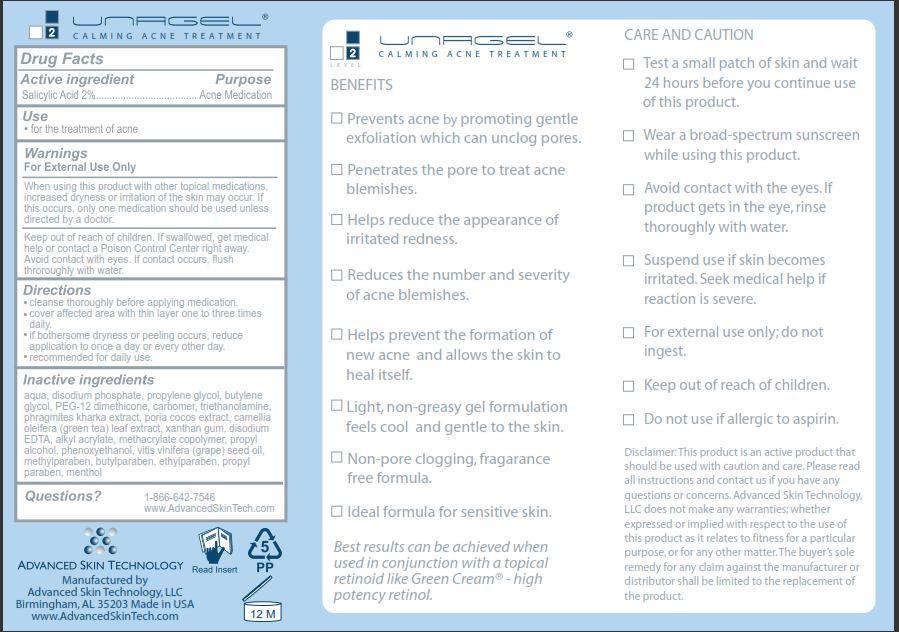
UNAGEL- salicylic acid cream
Advanced Skin Technology, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Unagel
Warnings
For External Use Only
When using this product with other topical medications, increased dryness or irritation of the skin may occur. If this occurs, only one medication should be used unless directed by a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. Avoid contact with eyes. If contact occurs, flush thoroughly with water.
Directions
- cleanse thoroughly before applying medication.
- cover affected area with thin layer one to three times daily.
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- recommended for daily use.
Inactive ingredients
aqua, disodium phosphate, propylene glycol, butylene glycol, PEG-12 dimethicone, carbomer, triethanolamine, phragmites, kharka extract, poria cocos extract, camellia oleifera (green tea) leaf extract, xanthan gum, disodium EDTA, alkyl acrylate, methacrylate copolymer, propyl alcohol, phenoxyethanol, vitis vinifera (grape) seed oil, methylparaben, butylparaben, ethylparaben, propyl paraben, menthol
BENEFITS
Prevents acne by promoting gentle exfoliation which can unclog pores.
Penetrates the pore to treat acne blemishes.
Helps reduce the appearance of irritated redness.
Reduces the number and severity of acne blemishes.
Helps prevent the formation of new acne and allows the skin to heal itself.
Light, non-greasy gel formulation feels cool and gentle to the skin.
Non-pore clogging, fragrance free formula.
Ideal formula for sensitive skin.
Best results can be achieved when used in conjunction with a topical retinoid like Green Cream - high potency retinol
CARE AND CAUTION
Test a small patch of skin and wait 24 hours before you continue use of this product.
Wear a broad-spectrum sunscreen while using this product.
Avoid contact with the eyes. If product gets in the eye, rinse thoroughly with water.
Suspend use if skin becomes irritates. Seek medical help if reaction is severe.
For external use only; do not ingest.
Keep out of reach of children.
Do not use if allergic to aspirin.
Disclaimer: This product is an active product that should be used with caution and care. Please read all instructions and contact us if you have any questions or concerns. Advanced Skin Technology, LLC does not make any warranties; whether expressed or implied with respect to the use of this product as it relates to fitness for a particular purpose, or for any other matter. The buyer's sole remedy for any claim against the manufacturer or distributor shall be limited to the replacement of the product.

| UNAGEL
salicylic acid cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Advanced Skin Technology, LLC (130977940) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Advanced Skin Technology, LLC | 130977940 | manufacture(52205-001) | |