PUREFORCE- benzalkonium solution
Ecolab Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
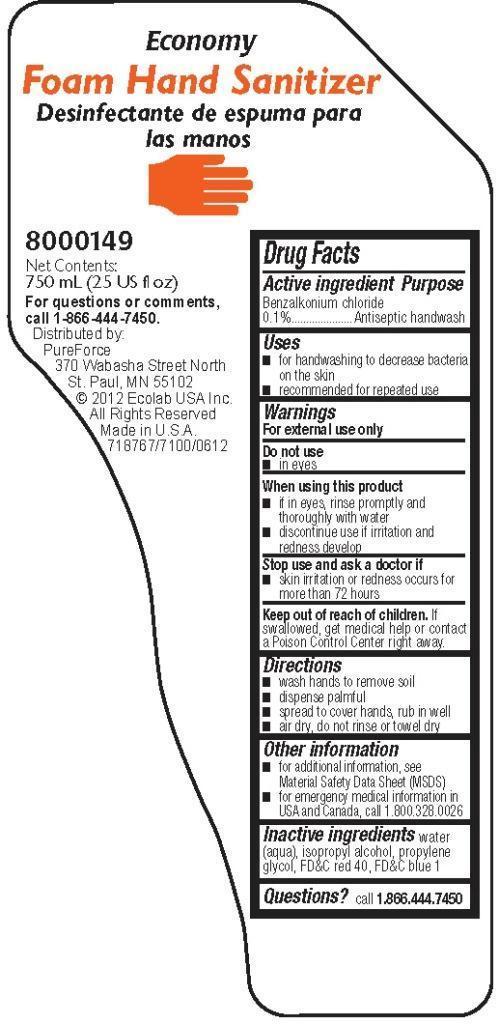
Drug Facts
Warnings
For external use only
Directions
- wash hands to remove soil
- dispense palmful
- spread to cover hands, rub in well
- air dry, do not rinse or towel dry
Other information
- for additional infromation, see Material Safety Data Sheet (MSDS)
- for emergency medical information in USA and Canada, call 1.800.328.0026
Principal display panel and representative label
Economy
Foam Hand Sanitizer
8000149
Net Contents:
750 ml (25 US fl oz)
For questions or comments, call 1-866-444-7450
Distributed by:
PureForce
370 Wabasha Street North
St. Paul, MN 55102
© 2012 Ecolab USA Inc.
All Rights Reserved
Made in U.S.A.
718767/7100/0612
| PUREFORCE
benzalkonium solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Ecolab Inc. (006154611) |
Revised: 2/2018
Document Id: 9ac5af25-1822-4733-99e8-9aa1c7b9a0c1
Set id: a687ebcc-7d29-4d92-b5a0-f4a184f8f8d3
Version: 2
Effective Time: 20180216
Ecolab Inc.