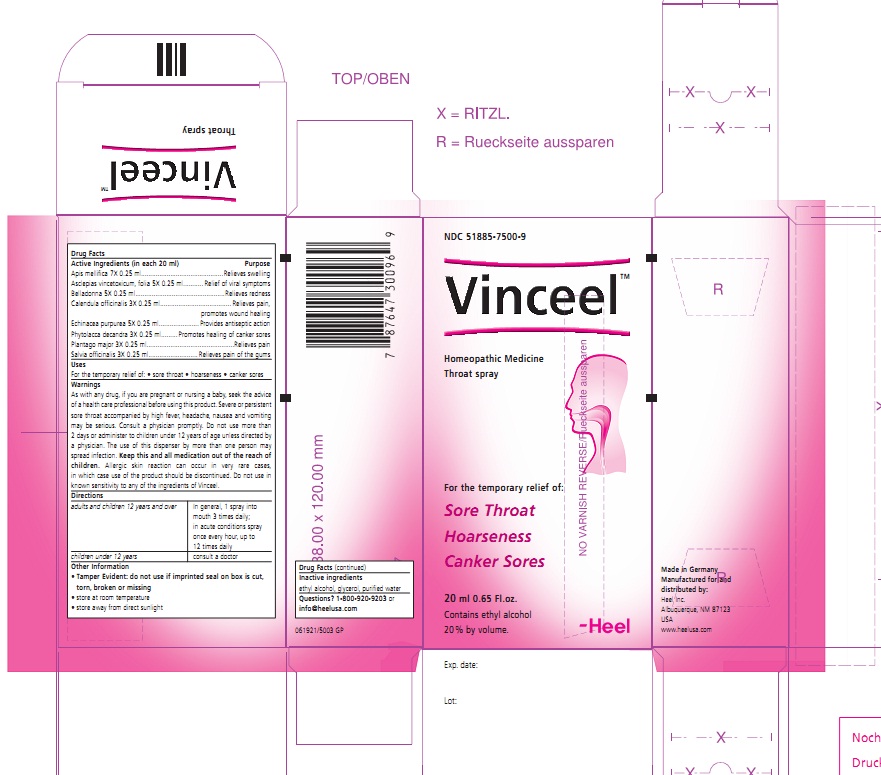
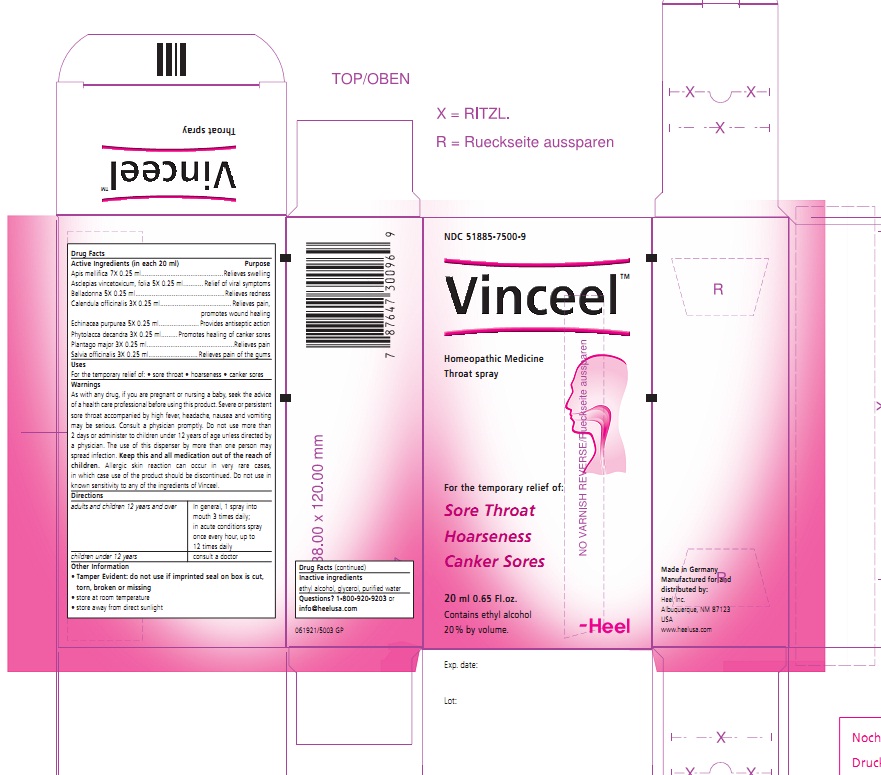
Label: VINCEEL- apis mellifera and cynanchum vincetoxicum leaf and atropa belladonna and calendula officinalis flowering top and echinacea purpurea and phytolacca americana root and plantago major and sage spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 51885-7500-9 - Packager: Biologische Heilmittel Heel
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 5, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PURPOSE
Apis mellifica 7X 0.25 ml....................................Relieves swelling
Asclepias vincetoxicum , folia 5X 0.25 ml............Relief of viral symptoms
Belladonna 5X 0.25 ml........................................Relieves redness
Calendula officinalis 3X 0.25 ml...........................Relieves pain, promotes wound healing
Echinacea purpurea 5X 0.25 ml...........................Provides antiseptic action
Phytolacca decandra 3X 0.25 ml.........................Promotes healing of canker sores
Plantago major 3X 0.25 ml..................................Relieves pain
Salvia officinalis 3X 0.25 ml................................Relieves pain of the gums
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS AND USAGE
-
WARNINGS
As with any drug, if you are pregnant or nursing a baby, seek the advise of a health care professional before using this product. Severe or persistent sore throat accompanied by high fever, headache, nausea and vomiting may be serious. Consult a physician promptly. Do not use more than 2 days or administer to children under 12 years of age unless directed by a physician. The use of this dispenser by more than one person may spread infection. Keep this and all medication out of the reach of children. Allergic skin reaction can occur in vary rare cases, in which case use of the product should be discontinued. Do not use in known sensitivity to any of the ingredients of Vinceel.
- DOSAGE AND ADMINISTRATION
- ACTIVE INGREDIENTS
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VINCEEL
apis mellifera and cynanchum vincetoxicum leaf and atropa belladonna and calendula officinalis flowering top and echinacea purpurea and phytolacca americana root and plantago major and sage sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51885-7500 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 7 [hp_X] in 20 mL CYNANCHUM VINCETOXICUM LEAF (UNII: 9WLP0FA6U4) (CYNANCHUM VINCETOXICUM LEAF - UNII:9WLP0FA6U4) CYNANCHUM VINCETOXICUM LEAF 5 [hp_X] in 20 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 5 [hp_X] in 20 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 3 [hp_X] in 20 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 5 [hp_X] in 20 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 3 [hp_X] in 20 mL PLANTAGO MAJOR (UNII: W2469WNO6U) (PLANTAGO MAJOR - UNII:W2469WNO6U) PLANTAGO MAJOR 3 [hp_X] in 20 mL SAGE (UNII: 065C5D077J) (SAGE - UNII:065C5D077J) SAGE 3 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51885-7500-9 1 in 1 CARTON 1 20 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/31/2006 Labeler - Biologische Heilmittel Heel (315635359)