FOAMING HAND SANITIZER V- alcohol solution
FOAMING HAND SANITIZER MM- alcohol solution
HAND SANITIZER- alcohol solution
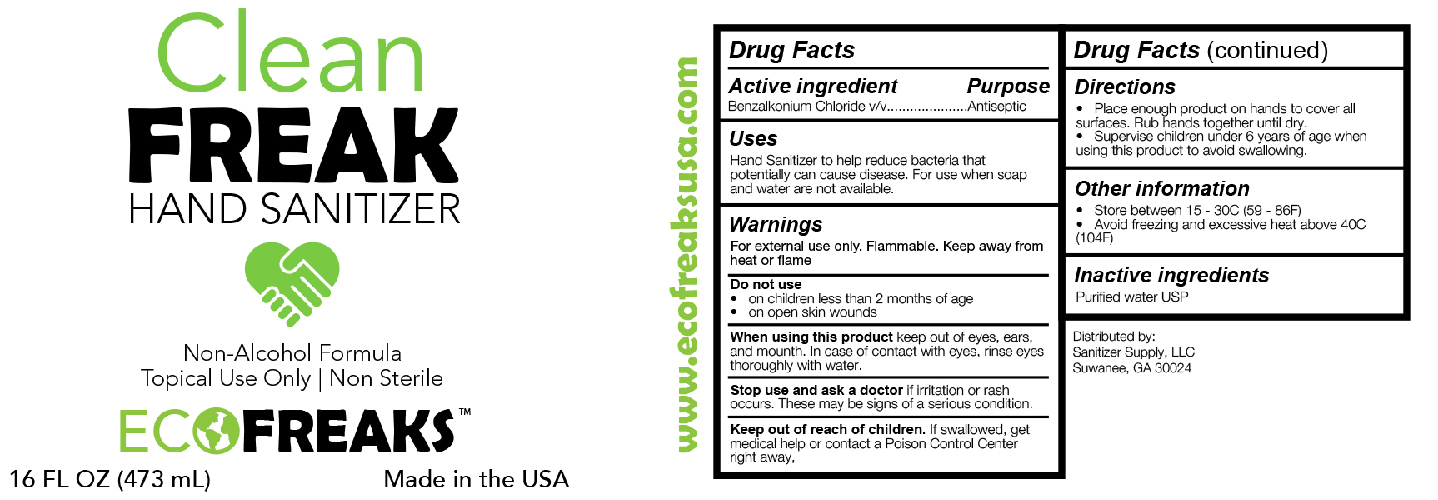
ECOFREAKS NON-ALCOHOL SANITIZER- benzalkonium chloride solution
ECOFREAKS HAND SANITIZER- alcohol solution
The Millennium Mat Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
This is a hand sanitizer manufactured according to the
Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (CoViD-19); Guidance for Industry.
The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation) consistent with World Health Organization (WHO) recommendations:
- Alcohol (ethanol) (USP or Food Chemical Codex (FCC) grade) (80%, volume/volume (v/v)) in an aqueous solution denatured according to Alcohol and Tobacco Tax and Trade Bureau regulations in 27 CFR part 20.
- Glycerol (1.45% v/v).
- Hydrogen peroxide (0.125% v/v).
- Sterile distilled water or boiled cold water.
The firm does not add other active or inactive ingredients. Different or additional ingredients may impact the quality and potency of the product.
Active Ingredient(s)
Alcohol 80% v/v. Purpose: Antiseptic
Purpose
Antiseptic, Hand Sanitizer
Use
Hand Sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
Warnings
For external use only. Flammable. Keep away from heat or flame
Do not use
- in children less than 2 months of age
- on open skin wounds
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Place enough product on hands to cover all surfaces. Rub hands together until dry.
- Supervise children under 6 years of age when using this product to avoid swallowing.
Other information
- Store between 15-30C (59-86F)
- Avoid freezing and excessive heat above 40C (104F)
Inactive ingredients
glycerin, hydrogen peroxide, purified water USP
Package Label - Principal Display Panel
75555-001-01: 2mL Hand Sanitizer Packet

75555-001-02: 125ct Box of 2mL Hand Sanitizer Packets

75555-001-03: 100mL Hand Sanitizer Pouch

75555-001-08: 8oz Hand Sanitizer Bottle

75555-001-12: 375mL Hand Sanitizer Bottle

75555-001-16: 16oz Hand Sanitizer Bottle

75555-001-32: 32oz Hand Sanitizer Bottle

75555-001-98: 55gal Drum Hand Sanitizer

75555-001-99: 1gal Hand Sanitizer Bottle

75555-002-17: 500mL Hand Sanitizer Bag

75555-003-17: 500mL Foaming Hand Sanitizer Bag

75555-010-16: 16oz Bottle Unscented Hand Sanitizer Gel

75555-011-16: 16oz Bottle Orange Scent Hand Sanitizer Gel

75555-110-16: 16oz Bottle Non-Alcohol Hand Sanitizer
75555-110-90: 10L Cannister 1000 Wipes BZK















