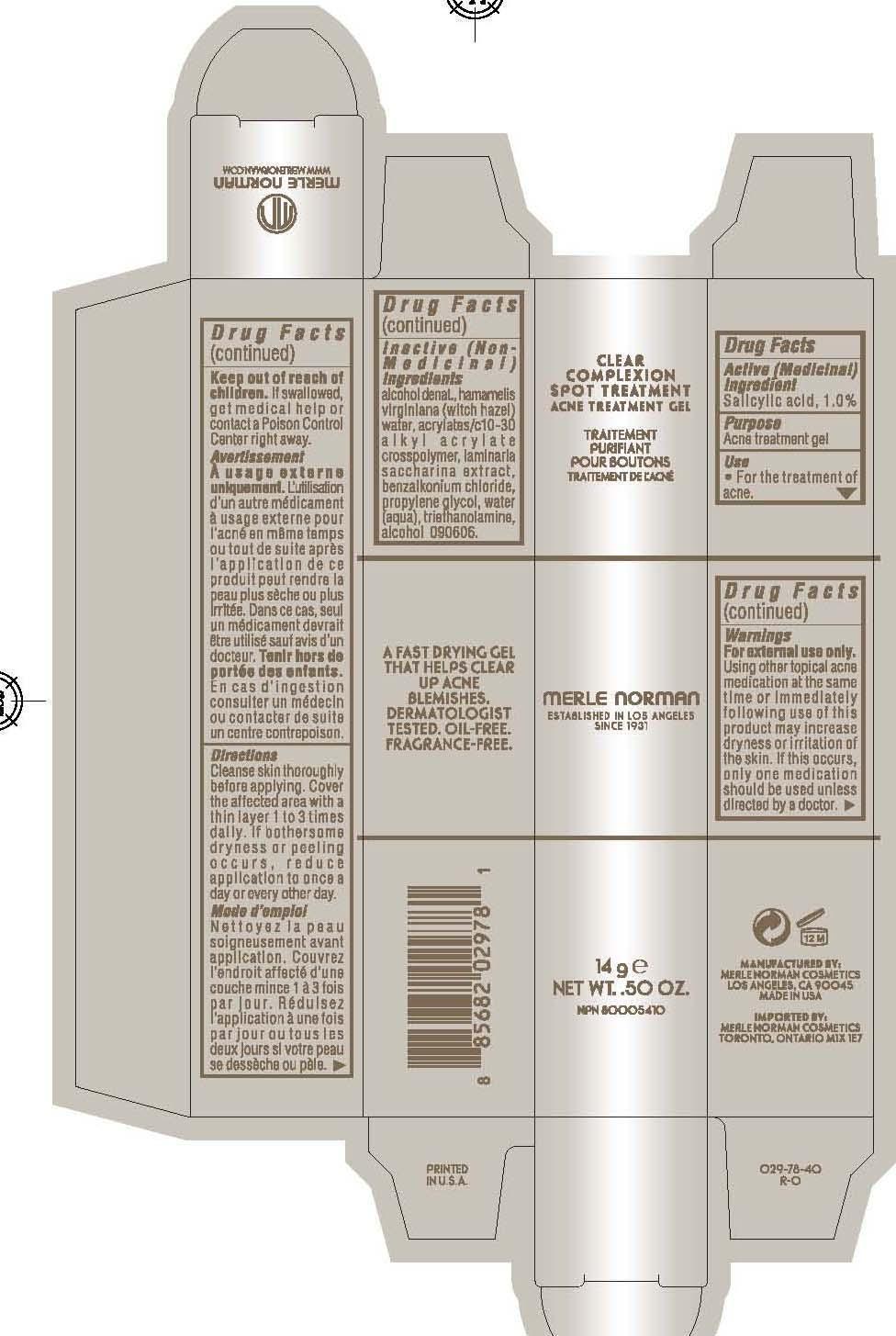
CLEAR COMPLEXION SPOT TREATMENT MERLE NORMAN- salicylic acid gel
Merle Norman Cosmetics, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Merle Norman Clear Complexion Spot Treatment
Active Ingredient Purpose
Salicylic Acid 1.0% Acne treatment gel
using other topical acne medication at the same time or immediately following the use of this product may increase dryness or irritation of the skin. if this occurs, only one medication should be used unless directed by a doctor.
| CLEAR COMPLEXION SPOT TREATMENT
MERLE NORMAN
salicylic acid gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Merle Norman Cosmetics, Inc (008479388) |
| Registrant - Merle Norman Cosmetics, Inc (008479388) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Merle Norman Cosmetics, Inc | 008479388 | manufacture(57627-100) | |
Revised: 2/2018
Document Id: 5f577aad-4459-465f-ab71-221ea6638ea4
Set id: a387dcf1-d822-4845-8826-8c130fe92b5f
Version: 15
Effective Time: 20180228
Merle Norman Cosmetics, Inc