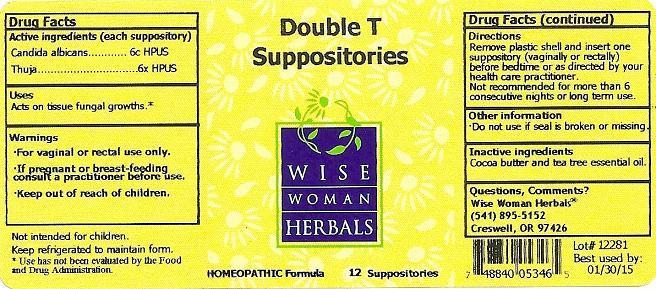
DOUBLE T- candida albicans, thuja occidentalis suppository
Wise Woman Herbals
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Directions
Remove plastic shell and insert one suppository (vaginally or rectally) before bedtime or as direected by your health care practitioner.
Not recommended for more than 6 consecutive nights or long term use.
| DOUBLE T
candida albicans, thuja occidentalis suppository |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Wise Woman Herbals (805908183) |
Revised: 2/2018
Document Id: 3f7a5600-ca0d-4bd5-83f1-d9e395f09d39
Set id: a31d8fbc-5d4a-42c2-9bc8-52c85e947213
Version: 4
Effective Time: 20180207
Wise Woman Herbals