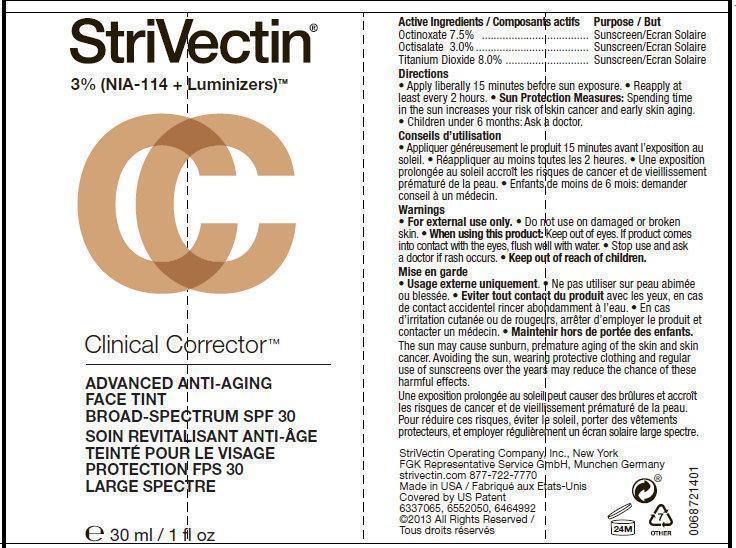
STRIVECTIN CLINICAL CORRECTOR ADVANCED ANTI-AGING FACE TINT LIGHT- octinoxate, octisalate, titanium dioxide cream
Strivectin Operating Company, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
StriVectin - Clinical Corrector ADVANCED ANTI-AGING FACE TINT LIGHT (76147-210), DELIST
Directions
• apply liberally 15 minutes before sun exposure
• reapply at least every two hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m.- 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months of age: ask a doctor
Inactive Ingredients
Aqua (Water, Eau), Cyclopentasiloxane, Butylene Glycol, PEG-10 Dimethicone, Myristyl Nicotinate, Isododecane, Phenyl Trimethicone, Aluminum Hydroxide, Stearic Acid, Tocopherol, Tocopheryl Acetate, Tetrahexyldecyl Ascorbate, Retinyl Palmitate, Sodium Hyaluronate, Glycyrrhiza Glabra (Licorice) Root Extract, Olive Glycerides, Ceramide 3, Caprylyl Glycol, Polyamide-5, Nylon-12, Bis-PEG/PPG-14/14 Dimethicone, Boron Nitride, Disteardimonium Hectorite, Sodium Dehydroacetate, Methicone, Triethosycaprylylsilane, Sodium Chloride, Hexylene Glycol, Mica, Disodium EDTA, Phenoxyethanol. May Contain +/-: CI 77891 (Titanium Dioxide), CI 77491/77492/77499 (Iron Oxides)
| STRIVECTIN CLINICAL CORRECTOR ADVANCED ANTI-AGING FACE TINT LIGHT
octinoxate, octisalate, titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Strivectin Operating Company, Inc. (832343722) |