EXCENEL RTU STERILE- ceftiofur hydrochloride injection, suspension
Zoetis Inc.
----------
Excenel® RTU
brand of ceftiofur hydrochloride sterile suspension
For intramuscular and subcutaneous use in cattle and intramuscular use in swine. This product may be used in lactating dairy cattle.
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION
EXCENEL RTU Sterile Suspension is a ready to use formulation that contains the hydrochloride salt of ceftiofur, which is a broad spectrum cephalosporin antibiotic.
Each mL of this ready-to-use sterile suspension contains ceftiofur hydrochloride equivalent to 50 mg ceftiofur, 0.50 mg phospholipon, 1.5 mg sorbitan monooleate, 2.5 mg sterile water for injection, and cottonseed oil.
Structure:
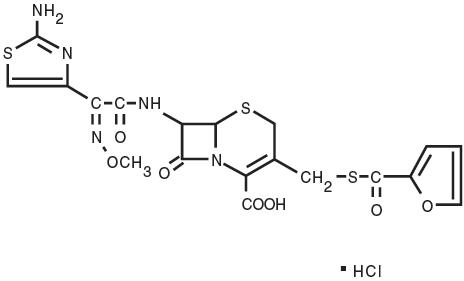
Figure 1
Chemical Name of Ceftiofur Hydrochloride: 5-Thia-1-azabicyclo[4,2.0]oct-2-ene-2-carboxylic acid, 7-[[(2-amino-4-thiazolyl)(methoxyimino)-acetyl]amino]-3-[[(2-furanylcarbonyl) thio]methyl]-8-oxo-,hydrochloride salt [6R-[6α,7β(Z)]]-
INDICATIONS
Swine: EXCENEL RTU Sterile Suspension is indicated for treatment/control of swine bacterial respiratory disease (swine bacterial pneumonia) associated with Actinobacillus (Haemophilus) pleuropneumoniae, Pasteurella multocida, Salmonella choleraesuis and Streptococcus suis.
Cattle: EXCENEL RTU Sterile Suspension is indicated for treatment of the following bacterial diseases:
- Bovine respiratory disease (BRD, shipping fever, pneumonia) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni.
- Acute bovine interdigital necrobacillosis (foot rot, pododermatitis) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus.
- Acute metritis (0 to 14 days post-partum) associated with bacterial organisms susceptible to ceftiofur.
DOSAGE AND ADMINISTRATION
Shake well before using.
Swine: Administer intramuscularly at a dosage of 1.36 to 2.27 mg ceftiofur equivalents/lb (3.0 to 5.0 mg/kg) BW (1 mL of sterile suspension per 22 to 37 lb BW). Treatment should be repeated at 24 h intervals for a total of three consecutive days.
Cattle:
— For bovine respiratory disease and acute bovine interdigital necrobacillosis: administer by intramuscular or subcutaneous administration at the dosage of 0.5 to 1.0 mg ceftiofur equivalents/lb (1.1 to 2.2 mg/kg) BW (1 to 2 mL sterile suspension per 100 lb BW). Administer daily at 24 h intervals for a total of three consecutive days. Additional treatments may be administered on Days 4 and 5 for animals which do not show a satisfactory response (not recovered) after the initial three treatments. In addition, for BRD only, administer intramuscularly or subcutaneously 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW every other day on Days 1 and 3 (48 h interval). Do not inject more than 15 mL per injection site.
Selection of dosage level (0.5 to 1.0 mg/lb) and regimen/duration (daily or every other day for BRD only) should be based on an assessment of the severity of disease, pathogen susceptibility and clinical response.
— For acute post-partum metritis: administer by intramuscular or subcutaneous administration at the dosage of 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW (2 mL sterile suspension per 100 lb BW). Administer at 24 h intervals for five consecutive days. Do not inject more than 15 mL per injection site.
CONTRAINDICATIONS
As with all drugs, the use of EXCENEL RTU Sterile Suspension is contraindicated in animals previously found to be hypersensitive to the drug.
WARNINGS
NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN.
Penicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposures to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions in some individuals. Repeated or prolonged exposure may lead to sensitization. Avoid direct contact of the product with the skin, eyes, mouth, and clothing.
Persons with a known hypersensitivity to penicillin or cephalosporins should avoid exposure to this product.
In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. If allergic reaction occurs (e.g., skin rash, hives, difficult breathing), seek medical attention.
The material safety data sheet contains more detailed occupational safety information. To obtain a material safety data sheet (MSDS) please call 1-800-733-5500 or to report any adverse event please call 1-888-963-8471.
RESIDUE WARNINGS:
Swine: When used according to label indications, dosage, and route of administration, treated swine must not be slaughtered for 4 days following the last treatment. Use of dosages in excess of those indicated or by unapproved routes of administration may result in illegal residues in edible tissues.
Cattle: When used according to label indications, dosage and route of administration, treated cattle must not be slaughtered for 3 days following the last treatment. When used according to label indications, dosage and route of administration, a milk discard time is not required. Uses of dosages in excess of those indicated or by unapproved routes of administration, such as intramammary, may result in illegal residues in edible tissues and/or milk. A withdrawal period has not been established in pre-ruminating calves. Do not use in calves to be processed for veal.
PRECAUTIONS
The effects of ceftiofur on cattle and swine reproductive performance, pregnancy, and lactation have not been determined.
Swine: Areas of discoloration associated with the injection site at time periods of 11 days or less may result in trim-out of edible tissues at slaughter. The safety of ceftiofur has not been demonstrated for pregnant swine or swine intended for breeding.
Cattle: Following intramuscular or subcutaneous administration in the neck, areas of discoloration at the site may persist beyond 11 days resulting in trim loss of edible tissues at slaughter. Following intramuscular administration in the rear leg, areas of discoloration at the injection site may persist beyond 28 days resulting in trim loss of edible tissues at slaughter.
CLINICAL PHARMACOLOGY
Swine: Ceftiofur administered as either ceftiofur sodium or ceftiofur hydrochloride is metabolized rapidly to desfuroylceftiofur, the primary metabolite. Administration of ceftiofur to swine as either the sodium or hydrochloride salt provides effective concentrations of ceftiofur and desfuroylceftiofur metabolites in plasma above the MIC90 for the labeled pathogens: Actinobacillus pleuropneumoniae, Pasteurella multocida, Streptococcus suis and Salmonella choleraesuis for the 24 hour (h) period between the dosing intervals. The MIC90 for Salmonella choleraesuis (1.0 µg/mL) is higher than the other three pathogens and plasma concentrations exceed this value for the entire dosing interval only after the 2.27 mg/lb (5.0 mg/kg) body weight (BW) dose.
Comparative Bioavailability Summary
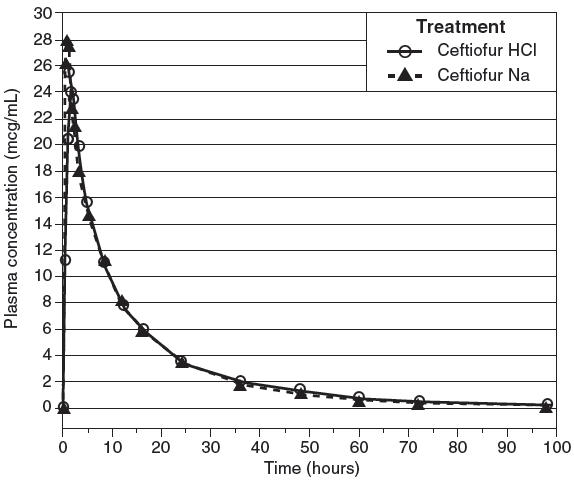
Comparable plasma concentrations of ceftiofur administered as ceftiofur hydrochloride sterile suspension (EXCENEL RTU Sterile Suspension) or ceftiofur sodium sterile solution (NAXCEL® Sterile Powder) were demonstrated after intramuscular administration of 2.27 mg ceftiofur equivalents/lb (5.0 mg/kg) BW. See Table 1 and Figure 2.
| Ceftiofur hydrochloride | Ceftiofur sodium | |
|---|---|---|
| Definitions: | ||
| Cmax – maximum plasma concentration in µg/mL. | ||
| tmax – the time after initial injection to when Cmax occurs, measured in hours. | ||
| AUC0-LOQ – the area under the plasma concentration vs. time curve from time of injection to the limit of quantitation of the assay (0.15 µg/mL). | ||
| t1/2 – the plasma half life of the drug in hours. | ||
| C24 h – the concentration of drug at 24 h after administration. | ||
| C72 h – the concentration of drug at 72 h after administration. | ||
| t>0.2 – the time (in hours) plasma concentrations remain above 0.2 µg/mL. | ||
|
||
| Cmaxµ g/mL: | 26.1 ± 5.02 | 29.2 ± 5.01 |
| tmax h: | 0.66 – 2.0 (range) | 0.33 – 2.0 (range) |
| AUC0-LOQ µg∙h/mL: | 321 ± 50.2 | 314 ± 55.1 |
| t1/2 h: | 16.2 ± 1.55 | 14.0 ± 1.23 |
| C24 hµg/mL: | 3.45 ± 0.431 | 3.53 ± 0.791 |
| C72 hµg/mL: | 0.518 ± 0.126 | 0.407 ± 0.0675 |
| t>0.2 h: | 93.8 ± 7.98 | 85.0 ± 7.71 |
Figure 2. Swine plasma concentrations of ceftiofur and desfuroylceftiofur metabolites after EXCENEL RTU Sterile Suspension (ceftiofur hydrochloride sterile suspension, 50 mg/mL) or NAXCEL Sterile Powder (ceftiofur sodium sterile powder, 50 mg/mL) were administered intramuscularly at 2.27 mg ceftiofur equivalents/lb (5.0 mg/kg) BW.
Concentrations of total ceftiofur in the lungs of pigs administered radiolabeled ceftiofur at 2.27 or 3.41 mg ceftiofur equivalents/lb (5.0 or 7.5 mg/kg) BW 12 h after the last of three daily intramuscular injections at 24 h intervals averaged 3.66 and 5.63 µg/g.
Cattle: Ceftiofur administered as either ceftiofur sodium or ceftiofur hydrochloride is metabolized rapidly to desfuroylceftiofur, the primary metabolite. Administration of ceftiofur to cattle as either the sodium or hydrochloride salt provides effective concentrations of ceftiofur and desfuroylceftiofur metabolites in plasma above the MIC90 for the bovine respiratory disease (BRD) label pathogens Mannheimia haemolytica, Pasteurella multocida and Histophilus somni for at least 48 h. The relationship between plasma concentrations of ceftiofur and desfuroylceftiofur metabolites above the MIC90 in plasma and efficacy has not been established for the treatment of bovine interdigital necrobacillosis (foot rot) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus.
Comparative Bioavailability Summary
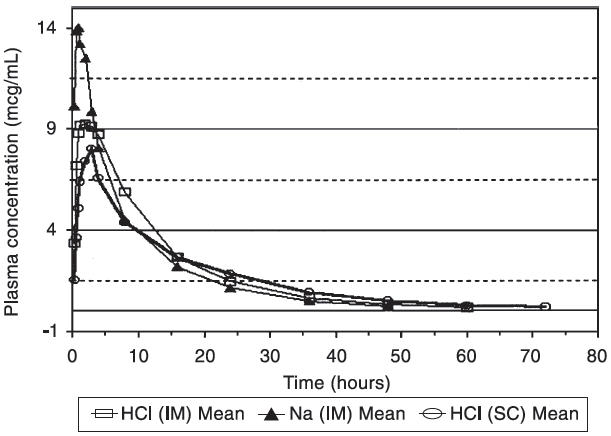
The comparability of plasma concentrations of ceftiofur following administration of ceftiofur hydrochloride sterile suspension (EXCENEL RTU Sterile Suspension) or ceftiofur sodium sterile solution (NAXCEL Sterile Powder) was demonstrated after intramuscular or subcutaneous administration of ceftiofur hydrochloride and intramuscular administration of ceftiofur sodium at 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW. See Table 2 and Figure 3.
| Ceftiofur hydrochloride | Ceftiofur sodium | ||
|---|---|---|---|
| IM | SC | IM* | |
| Definitions: | |||
| Cmax – maximum concentration of drug in plasma in µg/mL | |||
| tmax – the time after initial injection to when Cmax occurs, measured in hours | |||
| t>0.2 – the time (in hours) plasma drug concentrations remain above 0.2 µg/mL | |||
| AUC0-LOQ – the area under the plasma drug concentration vs. time curve from time of injection to the limit of quantitation of the assay (0.15 µg/mL) | |||
| t1/2 – the drug half life in plasma expressed in hours | |||
| C24 h – the plasma drug concentration 24 h after administration | |||
| C48 h – the plasma drug concentration 48 h after administration | |||
|
|||
| Cmax µg/mL | 11.0 ± 1.69 | 8.56 ± 1.89 | 14.4–16.5 |
| tmax h | 1–4 (range) | 1–5 (range) | 0.33–3.0 |
| t>0.2 h | 60.5 ± 6.27 | 51.0 ± 6.53 | 50.7–50.9 |
| AUC0-LOQ µg∙h/mL | 160 ± 30.7 | 95.4 ± 17.8 | 115–142 |
| t1/2 h | 12.0 ± 2.63 | 11.5 ± 2.57 | 9.50–11.1 |
| C24 h µg/mL | 1.47 ± 0.380 | 0.926 ± 0.257 | 0.86–1.16 |
| C48 h µg/mL | 0.340 ± 0.110 | 0.271 ± 0.086 | 0.250–0.268 |
Figure 3. Cattle plasma concentrations of ceftiofur and desfuroylceftiofur metabolites after EXCENEL RTU Sterile Suspension (ceftiofur hydrochloride sterile suspension, 50 mg/mL) was administered either intramuscularly or subcutaneously or NAXCEL Sterile Powder (ceftiofur sodium sterile powder, 50 mg/mL) was administered intramuscularly at 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW.
Total residues of ceftiofur were measured in the lungs of cattle administered radiolabeled ceftiofur at 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW at 24 h intervals for five consecutive days. Twelve h after the fifth injection of ceftiofur hydrochloride, total ceftiofur concentrations in the lung averaged 1.15 µg/g, while total ceftiofur concentrations in the lung 8 h after the fifth ceftiofur sodium injection averaged 1.18 µg/g.
CLINICAL MICROBIOLOGY
EXCENEL RTU Sterile Suspension is a ready to use formulation that contains the hydrochloride salt of ceftiofur, which is a broad spectrum cephalosporin antibiotic active against gram-positive and gram-negative bacteria including β-lactamase-producing strains. Like other cephalosporins, ceftiofur is bacteriocidal, in vitro, resulting in inhibition of cell wall synthesis.
Swine: Studies with ceftiofur have demonstrated in vitro and in vivo activity against gram-negative pathogens, including Actinobacillus (Haemophilus) pleuropneumoniae, Pasteurella multocida, Salmonella choleraesuis, and the gram-positive pathogen Streptococcus suis, all of which can be associated with swine bacterial respiratory disease – SRD (swine bacterial pneumonia). A summary of the minimum inhibitory concentration (MIC) values from SRD pathogens isolated from clinical field effectiveness studies is found in Table 3. Historic diagnostic laboratory MIC values for SRD pathogens from the US and Canada are found in Table 4.
Cattle: Studies with ceftiofur have demonstrated in vitro and in vivo activity against Mannheimia haemolytica, Pasteurella multocida and Histophilus somni, the three major pathogenic bacteria associated with bovine respiratory disease (BRD, pneumonia, shipping fever), and against Fusobacterium necrophorum and Bacteroides melaninogenicus, two of the major pathogenic anaerobic bacteria associated with acute bovine interdigital necrobacillosis (foot rot, pododermatitis). A summary of the MIC values for BRD and foot rot pathogens isolated from clinical field effectiveness studies is found in Table 3. Historic diagnostic MIC values for BRD and foot rot pathogens from the US and Canada are found in Table 4.
Antimicrobial Susceptibility
Summaries of MIC data are presented in Tables 3 and 4. Testing followed Clinical and Laboratory Standards Institute (CLSI) Guidelines.
| Animal | Organism | Number Tested | Date Tested | MIC90*
(µg/mL) | MIC Range (µg/mL) |
|---|---|---|---|---|---|
|
|||||
| Bovine |
Mannheimia haemolytica | 461 | 1988–1992 | 0.06 | ≤0.03–0.13 |
| Mannheimia haemolytica | 42 | 1993 | 0.015 | ≤0.003–0.03 | |
| Pasteurella multocida | 318 | 1988–1992 | 0.06 | ≤0.03–0.25 | |
| Pasteurella multocida | 48 | 1993 | ≤0.003 | ≤0.003–0.015 | |
| Histophilus somni | 109 | 1988–1992 | 0.06 | ≤0.03–0.13 | |
| Histophilus somni | 59 | 1993 | ≤0.0019 | no range | |
| Fusobacterium necrophorum | 17 | 1994 | ≤0.06 | no range | |
| Swine |
Actinobacillus pleuropn. | 83 | 1993 | ≤0.03 | ≤0.03–0.06 |
| Pasteurella multocida | 74 | 1993 | ≤0.03 | ≤0.03–0.06 | |
| Streptococcus suis | 94 | 1993 | 0.25 | ≤0.03–1.0 | |
| Salmonella choleraesuis | 50 | 1993 | 1.0 | 1.0–2.0 | |
| beta–hemolytic Streptococcus spp. | 24 | 1993 | ≤0.03 | ≤0.03–0.06 | |
| Actinobacillus suis | 77 | 1998 | 0.0078 | 0.0019–0.0078 | |
| Haemophilus parasuis | 76 | 1998 | 0.06 | 0.0039–0.25 | |
| Animal | Organism | Number Tested | Date Tested | MIC90†
(µg/mL) | MIC Range (µg/mL) |
|---|---|---|---|---|---|
| Bovine |
Mannheimia haemolytica | 110 | 1997–1998 | 0.06 | ≤0.03–0.25 |
| Mannheimia haemolytica | 139 | 1998–1999 | ≤0.03 | ≤0.03–0.5 | |
| Mannheimia haemolytica | 209 | 1999–2000 | ≤0.03 | ≤0.03–0.12 | |
| Mannheimia haemolytica | 189 | 2000–2001 | ≤0.03 | ≤0.03–0.12 | |
| Pasteurella multocida | 107 | 1997–1998 | ≤0.03 | ≤0.03–0.25 | |
| Pasteurella multocida | 181 | 1998–1999 | ≤0.03 | ≤0.03–0.5 | |
| Pasteurella multocida | 208 | 1999–2000 | ≤0.03 | ≤0.03–0.12 | |
| Pasteurella multocida | 259 | 2000–2001 | ≤0.03 | ≤0.03–0.12 | |
| Histophilus somni | 48 | 1997–1998 | ≤0.03 | ≤0.03–0.25 | |
| Histophilus somni | 87 | 1998–1999 | ≤0.03 | ≤0.03–0.125 | |
| Histophilus somni | 77 | 1999–2000 | ≤0.03 | ≤0.03–0.06 | |
| Histophilus somni | 129 | 2000–2001 | ≤0.03 | ≤0.03–0.12 | |
| Bacteroides fragilis group | 29 | 1994 | 16.0 | ≤0.06–>16.0 | |
| Bacteroides spp., non-fragilis group | 12 | 1994 | 16.0 | 0.13–>16.0 | |
| Peptostreptococcus anaerobius | 12 | 1994 | 2.0 | 0.13–2.0 | |
| Swine |
Actinobacillus pleuropn. | 97 | 1997–1998 | ≤0.03 | no range |
| Actinobacillus pleuropn. | 111 | 1998–1999 | ≤0.03 | ≤0.03–0.25 | |
| Actinobacillus pleuropn. | 126 | 1999–2000 | ≤0.03 | ≤0.03–0.06 | |
| Actinobacillus pleuropn. | 89 | 2000–2001 | ≤0.03 | ≤0.03–0.06 | |
| Pasteurella multocida | 114 | 1997–1998 | ≤0.03 | ≤0.03–1.0 | |
| Pasteurella multocida | 147 | 1998–1999 | ≤0.03 | ≤0.03–0.5 | |
| Pasteurella multocida | 173 | 1999–2000 | ≤0.03 | ≤0.03–0.06 | |
| Pasteurella multocida | 186 | 2000–2001 | ≤0.03 | ≤0.03–0.12 | |
| Streptococcus suis | 106 | 1997–1998 | 0.5 | ≤0.03–4.0 | |
| Streptococcus suis | 142 | 1998–1999 | 0.25 | ≤0.03–1.0 | |
| Streptococcus suis | 146 | 1999–2000 | 0.06 | ≤0.03–4.0 | |
| Streptococcus suis | 167 | 2000–2001 | 0.06 | ≤0.03–4.0 | |
| Salmonella choleraesuis | 96 | 1999–2000 | 1.0 | 0.03–>4.0 | |
| Salmonella choleraesuis | 101 | 2000–2001 | 1.0 | 0.5–2.0 | |
Based on the pharmacokinetic studies of ceftiofur in swine and cattle after a single intramuscular injection of 1.36 to 2.27 mg ceftiofur equivalents/lb (3.0 to 5.0 mg/kg) BW (swine) or 0.5 to 1.0 mg ceftiofur equivalents/lb (1.1 to 2.2 mg/kg) BW (cattle) and the MIC and disk (30 µg) diffusion data, the following breakpoints are recommended by CLSI.
| Zone Diameter (mm) | MIC (µg/mL) | Interpretation |
|---|---|---|
| ≥ 21 | ≤ 2.0 | (S) Susceptible |
| 18-20 | 4.0 | (I) Intermediate |
| ≤ 17 | ≥ 8.0 | (R) Resistant |
A report of "Susceptible" indicates that the pathogen is likely to be inhibited by generally achievable blood levels. A report of "Intermediate" is a technical buffer zone and isolates falling into this category should be retested. Alternatively the organism may be successfully treated if the infection is in a body site where drug is physiologically concentrated. A report of "Resistant" indicates that the achievable drug concentrations are unlikely to be inhibitory and other therapy should be selected.
Standardized procedures1 require the use of laboratory control organisms for both standardized diffusion techniques and standardized dilution techniques. The 30 µg ceftiofur sodium disk should give the following zone diameters and the ceftiofur sodium standard reference powder (or disk) should provide the following MIC values for the reference strain. Ceftiofur sodium disks or powder reference standard is appropriate for both ceftiofur salts.
| Organism name (ATCC No.) | Zone diameter*
(mm) | MIC range (µg/mL) |
|---|---|---|
|
||
| Escherichia coli (25922) | 26–31 | 0.25–1.0 |
| Staphylococcus aureus (29213) | — | 0.25–1.0 |
| Staphylococcus aureus (25923) | 27–31 | — |
| Pseudomonas aeruginosa (27853) | 14–18 | 16.0–64.0 |
| Actinobacillus pleuropneumoniae (27090) | 34–42† | 0.004–0.015‡ |
| Histophilus somni (700025) | 36–46† | 0.0005–0.004‡ |
- 1
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard – Second Edition. NCCLS document M31-A2. CLSI, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898, 2002.
CLINICAL EFFICACY
Cattle: In addition to demonstrating comparable plasma concentrations, the following clinical efficacy data are provided.
A clinical study was conducted to evaluate the efficacy of ceftiofur hydrochloride administered subcutaneously for the treatment of the bacterial component of BRD under natural field conditions. When uniform clinical signs of BRD were present, 60 cattle (111 to 207 kg) were randomly assigned to one of the following treatment groups: negative control or ceftiofur hydrochloride at 0.5 or 1.0 ceftiofur equivalents/lb (1.1 or 2.2 mg/kg) BW. Treatments were administered daily for three consecutive days. Cattle were evaluated daily and animals that died or were euthanatized were necropsied and the lung lesions scored. On Day 15, all surviving animals were euthanatized and necropsied and the lung lesions scored. Mortality rates were 65%, 10% and 5% for negative controls, 0.5 mg ceftiofur equivalents/lb and 1.0 mg ceftiofur equivalents/lb, (1.1 or 2.2 mg/kg) BW, respectively. Mortality rates for both ceftiofur hydrochloride treatment groups were lower than for negative controls (P < 0.0001). Rectal temperatures 24 h after third treatment were 104.0°F, 103.1°F and 102.8°F for negative controls, 0.5 mg/lb and 1.0 mg/lb (1.1 or 2.2 mg/kg) BW, respectively. The temperatures for both ceftiofur hydrochloride treatment groups were lower than the negative controls (P ≤ 0.05). Ceftiofur hydrochloride administered subcutaneously for three consecutive days at 0.5 or 1.0 mg ceftiofur equivalents/lb (1.1 or 2.2 mg/kg) BW is an effective treatment for the bacterial component of BRD.
A three-location clinical field study was conducted to evaluate the efficacy of ceftiofur hydrochloride administered intramuscularly daily for three days or every other day (Days 1 and 3) for the treatment of the bacterial component of naturally occurring BRD. When uniform signs of BRD were present, 360 beef crossbred cattle were randomly assigned to one of the following treatment groups: negative control, ceftiofur sodium at 0.5 mg ceftiofur equivalents/lb (1.1 mg/kg) BW daily for three days, ceftiofur hydrochloride at 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW daily for three days, or ceftiofur hydrochloride at 1.0 mg ceftiofur equivalents/lb BW on Days 1 and 3 (every other day). All treatments were administered intramuscularly. All ceftiofur treatment groups (hydrochloride and sodium) and treatment regimens (every day and every other day) significantly (P<0.05) reduced Day 4 rectal temperature as compared to the negative control. Clinical success on Days 10 and 28 and mortality to Day 28 were not different for the ceftiofur groups (hydrochloride and sodium) and treatment regimens (every day and every other day). The results of this study demonstrate that daily and every other day (Days 1 and 3) intramuscular administration of ceftiofur hydrochloride are effective treatment regimens for the bacterial component of BRD.
An eight location study was conducted under natural field conditions to evaluate the efficacy of ceftiofur hydrochloride for the treatment of acute post-partum metritis (0 to 14 days post-partum). When clinical signs of acute post-partum metritis (rectal temperature ≥103°F and fetid vaginal discharge) were observed, 361 lactating dairy cows were assigned randomly to treatment or negative control. Cattle were dosed either subcutaneously or intramuscularly, daily for five consecutive days. On days 1, 5 and 9 after the last day of dose administration, cows were evaluated for clinical signs of acute post-partum metritis. A cure was defined as rectal temperature <103°F and lack of fetid discharge. Cure rate for the 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW dose group was significantly improved relative to cure rate of the negative control on day 9. The results of this study demonstrate that ceftiofur hydrochloride administered daily for five consecutive days at a dose of 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW is an effective treatment for acute post-partum metritis.
ANIMAL SAFETY
Swine: Results from a five-day tolerance study in normal feeder pigs indicated that ceftiofur sodium was well tolerated when administered at 57 mg ceftiofur equivalents/lb (125 mg/kg) (more than 25 times the highest recommended daily dosage of 2.27 mg/lb (5.0 mg/kg)) BW for five consecutive days. Ceftiofur administered intramuscularly to pigs produced no overt adverse signs of toxicity.
To determine the safety margin in swine, a safety/toxicity study was conducted. Five barrows and five gilts per group were administered ceftiofur sodium intramuscularly at 0, 2.27, 6.81 and 11.36 mg ceftiofur equivalents/lb (0, 5, 15, 25 mg/kg) BW for 15 days. This is 0, 1, 3 and 5 times the highest recommended dose of 2.27 mg/lb (5.0 mg/kg) BW/day and 5 times the recommended treatment length of 3 days. There were no adverse systemic effects observed, indicating that ceftiofur has a wide margin of safety when injected intramuscularly into feeder pigs at the highest recommended dose of 2.27 mg ceftiofur equivalents/lb (5.0 mg/kg) BW daily for 3 days or at levels up to 5 times the highest recommended dose for 5 times the recommended length of treatment.
A separate study evaluated the injection site tissue tolerance of EXCENEL RTU (ceftiofur hydrochloride) in swine when administered intramuscularly in the neck at 1.36 and 2.27 mg ceftiofur equivalents/lb (3.0 to 5.0 mg/kg) BW. Animals were necropsied at intervals to permit evaluations at 12 h, and 3, 5, 7, 9, 11, 15, 20, and 25 days after last injection. Injection sites were evaluated grossly at necropsy. No apparent changes (swelling or inflammation) were observed clinically after 12 h post-injection. Areas of discoloration associated with the injection site were observed at time periods less than 11 days after last injection.
Cattle: Results from a five-day tolerance study in feeder calves indicated that ceftiofur sodium was well tolerated at 25 times (25 mg ceftiofur equivalents/lb {55 mg/kg} BW) the highest recommended dose of 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW for five consecutive days. Ceftiofur administered intramuscularly had no adverse systemic effects.
In a 15-day safety/toxicity study, five steer and five heifer calves per group were administered ceftiofur sodium intramuscularly at 0 (vehicle control), 1, 3, 5 and 10 times the highest recommended dose of 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW to determine the safety factor. There were no adverse systemic effects indicating that ceftiofur sodium has a wide margin of safety when injected intramuscularly into the feeder calves at 10 times (10 mg ceftiofur equivalents/lb {22 mg/kg} BW) the recommended dose for three times (15 days) the recommended length of treatment of three to five days. Local tissue tolerance to intramuscular injection of ceftiofur hydrochloride was evaluated in the following study.
Results from a tissue tolerance study indicated that ceftiofur hydrochloride was well tolerated and produced no systemic toxicity in cattle when administered intramuscularly in the neck and rear leg at a dose of 1.0 mg ceftiofur equivalents/lb (2.2 mg/kg) BW at each injection site. This represents a total dose per animal of 2.0 mg ceftiofur equivalents/lb (4.4 mg/kg) BW. Clinically noted changes (local swelling) at injection sites in the neck were very infrequent (2/48 sites) whereas noted changes in rear leg sites were more frequent (21/48 sites). These changes in the rear leg injection sites were generally evident on the day following injection and lasted from 1 to 11 days. At necropsy, injection sites were recognized by discoloration of the subcutaneous tissues and muscle that resolved in approximately 7 to 15 days in the neck and 19 to 28 days in the rear leg.
Results from another tissue tolerance study indicated that ceftiofur hydrochloride was well tolerated and produced no systemic toxicity to cattle when administered subcutaneously at 0.5 or 1.0 mg ceftiofur equivalents/lb (1.1 or 2.2 mg/kg) BW at 24 h intervals for 5 days. Mild and usually transient, clinically visible or palpable reactions (local swelling) were localized at the injection site. At necropsy, injection sites were routinely recognized by edema, limited increase in thickness and color changes of the subcutaneous tissue and/or fascial surface of underlying muscle. The fascial surface of the muscle was visibly affected in most cases through 9.5 days after injection. Underlying muscle mass was not involved. There were no apparent differences in tissue response to administration of ceftiofur hydrochloride at 0.5 or 1.0 mg ceftiofur equivalents/lb (1.1 or 2.2 mg/kg) BW.
TISSUE RESIDUE DEPLETION
Swine: Radiolabeled residue metabolism studies established tolerances for ceftiofur residues in swine kidney, liver, and muscle. These tolerances of ceftiofur residues are 0.25 ppm in kidney, 3.0 ppm in liver and 2.0 ppm in muscle.
A pivotal tissue residue decline study was conducted in swine. In this study, pigs received 2.27 mg of ceftiofur per lb body weight (5 mg of ceftiofur per kg body weight) per day for three consecutive days. Ceftiofur residues in tissues were less than the tolerances for ceftiofur residues in tissues such as kidney, liver and muscle by 4 days after dosing. These data collectively support a 4-day pre-slaughter withdrawal period in swine when used according to label directions.
Cattle: A radiolabeled residue metabolism study established tolerances for ceftiofur residues in cattle kidney, liver and muscle. These tolerances of ceftiofur residues are 0.4 ppm in kidney, 2.0 ppm in liver, 1.0 ppm in muscle, and 0.1 ppm in milk.
A pivotal tissue residue decline study was conducted in cattle. In this study, cattle received a subcutaneous injection of 1.0 mg of ceftiofur per lb body weight (2.2 mg of ceftiofur per kg body weight) for five consecutive days. Ceftiofur residues in tissues were less than the tolerances for ceftiofur residues in tissues such as kidney, liver and muscle by 3 days after dosing. These data collectively support a 3-day pre-slaughter withdrawal period in cattle when used according to label directions.
STORAGE CONDITIONS
Store at controlled room temperature 20° to 25° C (68° to 77° F). Shake well before using. Protect from freezing. Contents should be used within 28 days after the first dose is removed.
NADA #140-890, Approved by FDA
Revised: March 2013
Distributed by:
Zoetis Inc.
Kalamazoo, MI 49007
10981201
PRINCIPAL DISPLAY PANEL - 100 mL Vial Label
100 mL
EXCENEL® RTU
Sterile Suspension
ceftiofur hydrochloride sterile suspension
Equivalent to
50 mg per mL
ceftiofur
For intramuscular and subcutaneous injection in
cattle and intramuscular injection in swine.
This Product May Be Used In Lactating Dairy Cattle.
Caution: Federal (USA) law restricts this drug to use
by or on the order of a licensed veterinarian.
For Use in Animals Only
NADA #140-890, Approved by FDA
zoetis
11328802
1431000
LOT/EXP

PRINCIPAL DISPLAY PANEL - 250 mL Vial Label
250 mL
EXCENEL® RTU
Sterile Suspension
ceftiofur hydrochloride sterile suspension
Equivalent to
50 mg per mL
ceftiofur
For intramuscular and subcutaneous injection in cattle
and intramuscular injection in swine.
This Product May Be Used In Lactating Dairy Cattle.
Caution: Federal (USA) law restricts this drug to use by
or on the order of a licensed veterinarian.
For Use in Animals Only
NADA #140-890, Approved by FDA
zoetis
10981301
1531000
LOT/EXP
| EXCENEL
RTU STERILE
ceftiofur hydrochloride injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Zoetis Inc. (828851555) |