METROGEL
-
metronidazole gel
Galderma Laboratories, L.P.
----------
DESCRIPTION
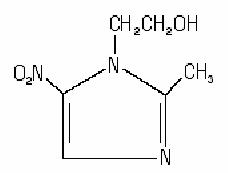
METROGEL® Topical Gel contains metronidazole, USP, at a concentration of 7.5 mg per gram (0.75%) in a gel consisting of carbomer 940, edetate disodium, methylparaben, propylene glycol, propylparaben, purified water, and sodium hydroxide. Metronidazole is classified therapeutically as an antiprotozoal and antibacterial agent. Chemically, metronidazole is named 2-methyl-5-nitro-1H-imidazole-1-ethanol and has the following structure:
CLINICAL PHARMACOLOGY
Bioavailability studies on the topical administration of 1 gram of METROGEL® Topical Gel (7.5 mg of metronidazole) to the face of 10 rosacea patients showed a maximum serum concentration of 66 nanograms per milliliter in one patient. This concentration is approximately 100 times less than concentrations afforded by a single 250 mg oral tablet. The serum metronidazole concentrations were below the detectable limits of the assay at the majority of time points in all patients. Three of the patients had no detectable serum concentrations of metronidazole at any time point. The mean dose of gel applied during clinical studies was 600 mg which represents 4.5 mg of metronidazole per application. Therefore, under normal usage levels, the formulation affords minimal serum concentrations of metronidazole. The mechanisms by which METROGEL® (metronidazole topical gel) Topical Gel acts in the treatment of rosacea are unknown, but appear to include an anti-inflammatory effect.
INDICATIONS AND USAGE
METROGEL® Topical Gel is indicated for topical application in the treatment of inflammatory papules and pustules of rosacea.
CONTRAINDICATIONS
METROGEL® Topical Gel is contraindicated in individuals with a history of hypersensitivity to metronidazole, parabens, or other ingredients of the formulation.
PRECAUTIONS
General: METROGEL® Topical Gel has been reported to cause tearing of the eyes. Therefore, contact with the eyes should be avoided. If a reaction suggesting local irritation occurs, patients should be directed to use the medication less frequently or discontinue use. Metronidazole is a nitroimidazole and should be used with care in patients with evidence of, or history of blood dyscrasia.
Information for patients: This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
Drug interactions: Oral metronidazole has been reported to potentiate the anticoagulant effect of coumarin and warfarin resulting in a prolongation of prothrombin time. The effect of topical metronidazole on prothrombin time is not known.
Carcinogenesis, mutagenesis, impairment of fertility: Metronidazole has shown evidence of carcinogenic activity in a number of studies involving chronic, oral administration in mice and rats but not in studies involving hamsters.
Metronidazole has shown evidence of mutagenic activity in several in vitro bacterial assay systems. In addition, a dose-response increase in the frequency of micronuclei was observed in mice after intraperitoneal injections and an increase in chromosome aberrations have been reported in patients with Crohn’s disease who were treated with 200-1200 mg/day of metronidazole for 1 to 24 months. However, no excess chromosomal aberrations in circulating human lymphocytes have been observed in patients treated for 8 months.
Pregnancy:Teratogenic effects Pregnancy category B: There has been no experience to date with the use of METROGEL® (metronidazole topical gel) Topical Gel in pregnant patients. Metronidazole crosses the placental barrier and enters the fetal circulation rapidly. No fetotoxicity was observed after oral metronidazole in rats or mice. However, because animal reproduction studies are not always predictive of human response and since oral metronidazole has been shown to be a carcinogen in some rodents, this drug should be used during pregnancy only if clearly needed.
Nursing mothers: After oral administration, metronidazole is secreted in breast milk in concentrations similar to those found in the plasma. Even though METROGEL® Topical Gel blood levels are significantly lower than those achieved after oral metronidazole, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE REACTIONS
The following adverse experiences have been reported with the topical use of metronidazole: burning, skin irritation, dryness, transient redness, metallic taste, tingling or numbness of extremities and nausea.
DOSAGE AND ADMINISTRATION
Apply and rub in a thin film of METROGEL® Topical Gel twice daily, morning and evening, to entire affected areas after washing.
Areas to be treated should be cleansed before application of METROGEL® (metronidazole topical gel) Topical Gel. Patients may use cosmetics after application of METROGEL® Topical Gel.
HOW SUPPLIED
METROGEL® (metronidazole topical gel) Topical Gel is supplied in a 45 g aluminum tube – NDC 0299-3835-45.
Storage conditions: STORE AT CONTROLLED ROOM TEMPERATURE: 68° to 77°F (20° to 25°C).
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
Manufactured by:
Galderma Production Canada, Inc.
Montreal, QC
H9X 3N7 Canada
Manufactured by:
DPT Laboratories, Ltd.
San Antonio, Texas 78215 USA
GALDERMA is a registered trademark.
www.metrogel.com
P50011-2
Revised: July 2004
PACKAGE LABEL
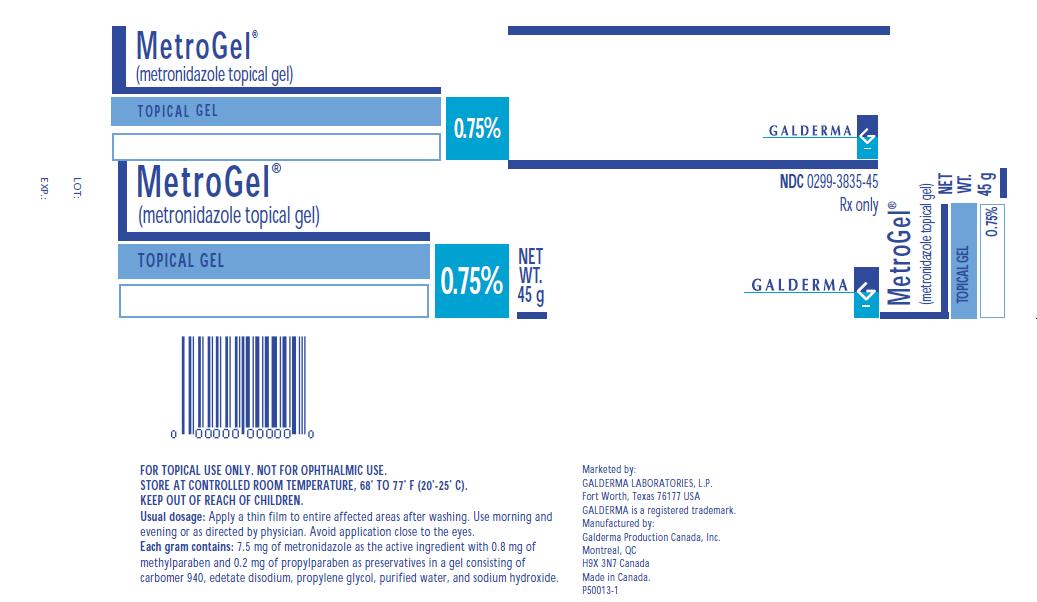
MetroGel®
(metronidazole topical gel)
TOPICAL GEL
0.75%
NET WT. 45 g
NDC 0299-3835-45
Rx only
GALDERMA
FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC USE.
STORE AT CONTROLLED ROOM TEMPERATURE, 68º TO 77° F (20°-25° C).
KEEP OUT OF REACH OF CHILDREN.
Usual dosage: Apply a thin film to entire affected areas after washing. Use morning and evening or as directed by physician. Avoid application close to the eyes.
Each gram contains: 7.5 mg of metronidazole as the active ingredient with 0.8 mg of methylparaben and 0.2 mg of propylparaben as preservatives in a gel consisting of carbomer 940, edetate disodium, propylene glycol, purified water, and sodium hydroxide.
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
GALDERMA is a registered trademark
Manufactured by:
Galderma Production Canada, Inc.
Montreal, QC
H9X 3N7 Canada
Made in Canada
P50013-1
LOT:
EXP.:
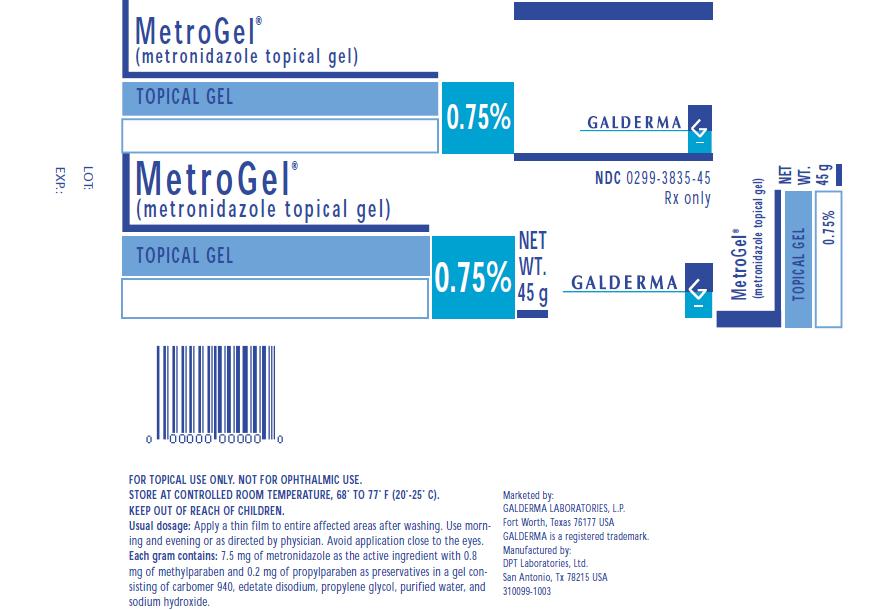
MetroGel®
(metronidazole topical gel)
TOPICAL GEL
0.75%
NET WT. 45 g
NDC 0299-3835-45
Rx only
GALDERMA
FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC USE.
STORE AT CONTROLLED ROOM TEMPERATURE, 68º TO 77° F (20°-25° C).
KEEP OUT OF REACH OF CHILDREN.
Usual dosage: Apply a thin film to entire affected areas after washing. Use morning and evening or as directed by physician. Avoid application close to the eyes.
Each gram contains: 7.5 mg of metronidazole as the active ingredient with 0.8 mg of methylparaben and 0.2 mg of propylparaben as preservatives in a gel consisting of carbomer 940, edetate disodium, propylene glycol, purified water, and sodium hydroxide.
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
GALDERMA is a registered trademark
Manufactured by:
DPT Laboratories, Ltd.
San Antonio, Tx 78215 USA
310099-1003
LOT:
EXP.:
| METROGEL
metronidazole gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019737 | 11/22/1988 | 09/30/2010 |
| Labeler - Galderma Laboratories, L.P. (047350186) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Galderma Production Canada Inc | 251676961 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| DPT Laboratories, Ltd. | 832224526 | MANUFACTURE | |
Revised: 01/2008 Galderma Laboratories, L.P.