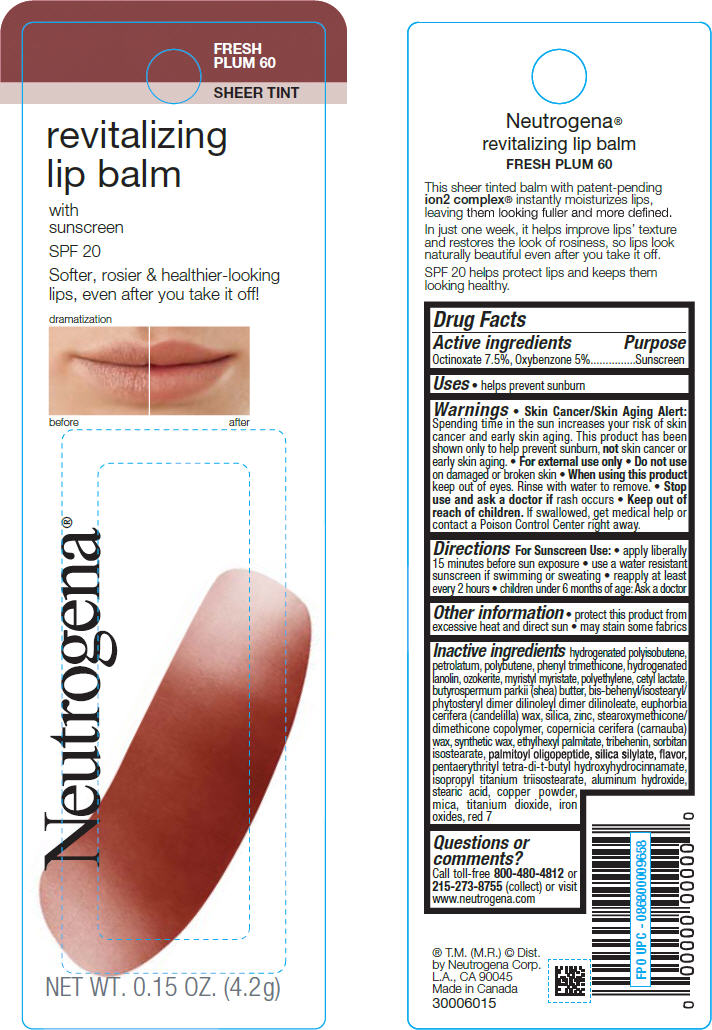
NEUTROGENA REVITALIZING LIP BALM SPF 20 - FRESH PLUM 60- octinoxate and oxybenzone lipstick
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Octinoxate 7.5%, Oxybenzone 5%
Warnings
-
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
-
For external use only
-
Do not use on damaged or broken skin
-
When using this product keep out of eyes. Rinse with water to remove
-
Stop use and ask a doctor if rash occurs
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
For Sunscreen Use
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months of age: Ask a doctor
Other information
- protect this product from excessive heat and direct sun
- may stain some fabrics
Inactive ingredients
Hydrogenated Polyisobutene, Petrolatum, Polybutene, Phenyl Trimethicone, Hydrogenated Lanolin, Ozokerite, Myristyl Myristate, Polyethylene, Cetyl Lactate, Butyrospermum Parkii (Shea) Butter, Bis-Behenyl/Isostearyl/Phytosteryl Dimer Dilinoleyl Dimer Dilinoleate, Euphorbia Cerifera (Candelilla) Wax, Silica, Zinc, Stearoxymethicone/Dimethicone Copolymer, Copernicia Cerifera (Carnauba) Wax, Synthetic Wax, Ethylhexyl Palmitate, Tribehenin, Sorbitan Isostearate, Palmitoyl Oligopeptide, Silica Silylate, Flavor, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Isopropyl Titanium Triisostearate, Aluminum Hydroxide, Stearic Acid, Copper Powder, Mica, Titanium Dioxide, Iron Oxides, Red 7
Questions or comments?
Call toll-free 800-480-4812 or 215-273-8755 (collect) or visit www.neutrogena.com
PRINCIPAL DISPLAY PANEL - 4.2 g Tube Container
FRESH
PLUM 60
SHEER TINT
revitalizing
lip balm
with
sunscreen
SPF20
Softer, rosier & healthier-looking
lips, even after you take it off!
Dramatization
before after
Neutrogena®
NET WT. 0.15 OZ. (4.2g)
Johnson & Johnson Consumer Inc.