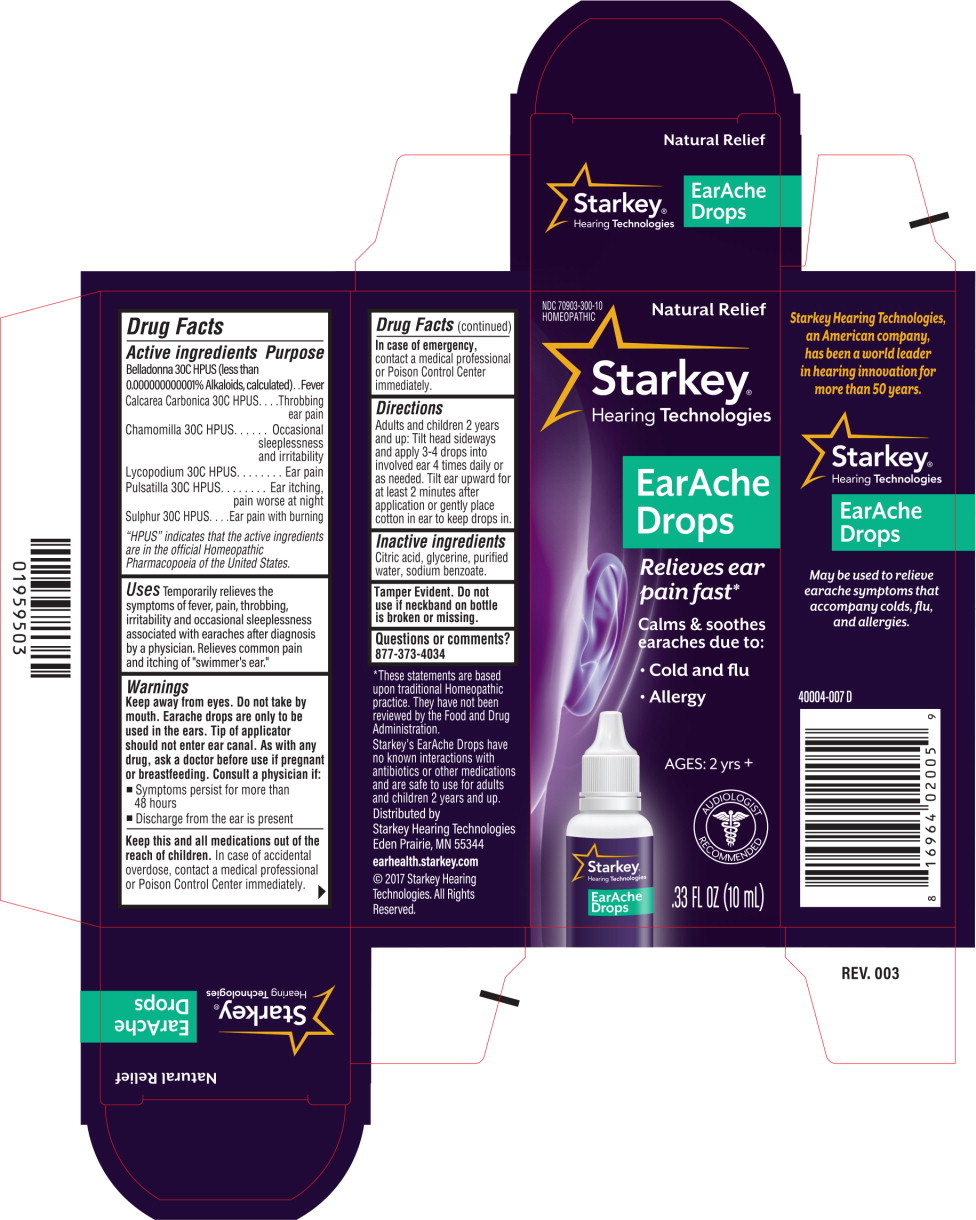
EARACHE- belladonna leaf, calcium carbonate, chamomile, lycopodium clavatum spore, anemone patens, and sulfur liquid
Starkey Hearing Technologies
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
EarAche Drops
Active ingredients
Belladonna 30C HPUS (less than 0.000000000001 % Alkaloids)
Calcarea Carbonica 30C HPUS
Chamomilla 30C HPUS
Lycopodium 30C HPUS
Pulsatilla 30C HPUS
Sulphur 30C HPUS
Purpose
Fever
Throbbing ear pain
Occasional sleeplessness and irritability
Ear pain
Ear itching, pain worse at night
Ear pain with burning
Uses
Temporarily relieves the symptoms of fever, pain, throbbing, irritability and sleeplessness associated with earaches after diagnosis by a physician. Relieves common pain and itching.
Warnings
Keep away from eyes. Do not take by mouth. Earache drops are only to be used in the ears. Tip of applicator should not enter ear canal.
Directions
Adults and children 2 years and up: Tilt head sideways and apply 3-4 drops into involved ear 4 times daily or as needed. Tilt ear upward for at least 2 minutes after application or gently place cotton in ear to keep drops in.
Inactive ingredients
Citric acid, glycerine, purified water, sodium benzoate.
Tamper Evident. Do not use if neckband on bottle is broken or missing.
| EARACHE
belladonna leaf, calcium carbonate, chamomile, lycopodium clavatum spore, anemone patens, and sulfur liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Starkey Hearing Technologies (055461479) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Standard Homeopathic Company | 008316655 | manufacture(70903-300) , pack(70903-300) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lifetech Resources | 622559110 | manufacture(70903-300) , pack(70903-300) , label(70903-300) | |