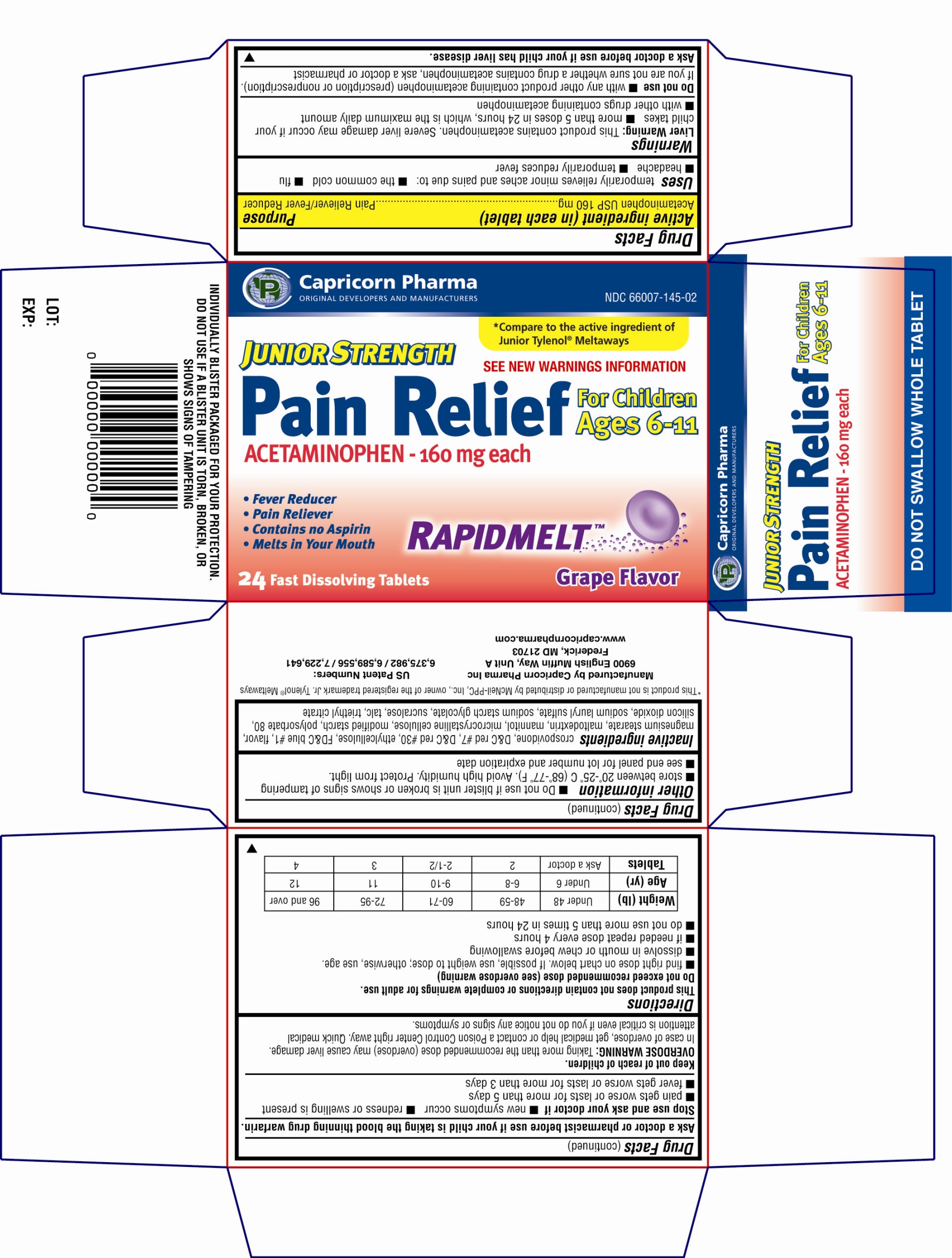
Label: JUNIOR STRENGTH PAIN RELIEF- acetaminophen tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 66007-145-01, 66007-145-02 - Packager: Capricorn Pharma Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 26, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(in each tablet)
- Purpose
- Uses
-
Warnings
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if your child takes:
■ more than 5 doses in 24 hours, which is maximum daily amount
■ with other drugs containing acetaminophen
Do not use
■ with any other product containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
-
Directions
This product does not contain directions or complete warnings for adult use.
Do not exceed recommended dose (see overdose warning)
■ find right dose on chart below. If possible, use weight to dose; otherwise, use age
■ dissolve in mouth or chew before swallowing
■ if needed, repeat dose every 4 hours
■ do not use more than 5 times in 24 hoursWeight (lb)
Under 48
48-59
60-71
72-95
96 and over
Age (yr)
Under 6
6-8
9-10
11
12
Tablets
Ask a doctor
2
2-1/2
3
4
- Other Information
- Inactive Ingredient
-
Principal Display Panel
Capricorn Pharma
ORIGINAL DEVELOPERS AND MANUFACTURERS
Compare to the active ingredient of Junior Tylenol Meltaways
JUNIOR STRENGTH
Pain Relief
ACETAMINOPHEN - 160mg each
For Children Ages 6-11
See new warnings information
* Fever Reducer
* Pain Reliever
* Contains no Aspirin
* Melts in your mouth
RAPIDMELT
24 Fast Dissolving Tablets
Grape Flavor
-
INGREDIENTS AND APPEARANCE
JUNIOR STRENGTH PAIN RELIEF
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66007-145 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg Product Characteristics Color purple (light purple/violet) Score 2 pieces Shape ROUND (Round Lozenge) Size 14mm Flavor GRAPE Imprint Code CP Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66007-145-02 4 in 1 CARTON 1 NDC:66007-145-01 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 07/26/2010 Labeler - Capricorn Pharma Inc. (041704524) Registrant - Capricorn Pharma Inc. (041704524) Establishment Name Address ID/FEI Business Operations Capricorn Pharma Inc. 041704524 manufacture