ATENDIA- menthol and lidocaine patch
OakLock, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
ATENDIA-menthol and lidocaine patch
DESCRIPTION
Atendia patch contains 4% lidocaine and 3% menthol in a localized topical dermal delivery system where lidocaine and menthol are applied to a polyester backing film coated with a drug-containing acrylic adhesive formulation, and covered with a removable paper release liner. The release liner is removed prior to application to the skin. The size of the patch is 3 in. x 5 in.
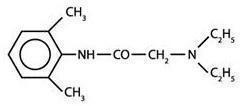
Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), has an octanol:water partition ratio of 43 at pH 7.4, and has the following structure:
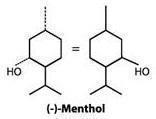
Menthol is chemically designated as [(1R,2S,5R)-2-isopropyl-5-methylcyclohexanol] and is an activating ligand for transient receptor potential cation channel subfamily M member 8 (TRPM8). It has the following structure:
Each adhesive patch contains 4mg of lidocaine and 3mg of menthol per 100mg.
INDICATIONS AND USAGE
Atendia Patch is indicated for the temporary relief of minor aches and muscle pains associated with arthritis, simple backache, strains, muscle soreness and stiffness. Atendia Patch is a topical anesthetic and external analgesic/counterirritant.
CONTRAINDICATIONS
Atendia Patch is contraindicated in patients with a known history of any sensitivity to menthol, local anesthetics of amide type, or to any other component of the product.
WARNINGS
- For external use only.
- Use only as directed.
- Avoid contact with eyes and mucous membranes.
- Do not cover with bandage.
- Do not use on wounds or damaged skin.
- Consult physician for children under 18.
- Do not use if you are allergic or have hypersensitivity to menthol, lidocaine, or any other component of the product.
- Stop use if allergic reaction occurs and consult a doctor if conditions worsen, symptoms persist for more than 7 days or clear up and occur again within a few days or rash, itching or excessive skin irritation occurs.
- Stop use if any application site reactions occur.
- KEEP OUT OF THE REACH OF CHILDREN.
DOSAGE AND ADMINISTRATION
Atendia Patch contains 4% lidocaine and 3% menthol. Patches may be cut into smaller sizes with scissors prior to removal of the release liner.
Clothing may be worn over the area of application.
INSTRUCTIONS FOR USE
- Clean and dry affected area.
- Cut open pouch and remove patch
- Remove protective film and apply on intact skin directly to area of pain
- Apply to affected area not more than 3 times daily
- Wash hands with soap after applying patch
- Reseal pouch containing unused patches.
DIRECTIONS
| Adults and children 18 years and over | Apply to affected area. Change patch 1 to 3 times daily. |
| Children under 18 years | Consult physician before use. |
HANDLING AND DISPOSAL
Hands should be washed after the handling of Atendia Patch, and eye contact with Atendia Patch should be avoided. Do not store patch outside the sealed protective pouch. Apply immediately after removal from the protective pouch. Fold used patches so that the adhesive sticks to itself and safely discard used patches or pieces of cut patches where children and pets cannot get to them. Atendia should be kept out of the reach of children.
HOW SUPPLIED
Atendia Patch is supplied in 3 re-sealable pouches each containing 5 patches.
NDC 69263-022-15
15 Patches per carton
Rx
| ATENDIA
menthol and lidocaine patch |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - OakLock, LLC (079559179) |
